MRgFUS in the treatment of MSK lesions: a review based on the experience of the University of L’Aquila, Italy
Introduction
High intensity focused ultrasound (HIFU), employed at experimental level during the last years, has currently gained an important clinical role in the musculoskeletal (MSK) field and in particular in the treatment of bone conditions. Current literature describes the employment of this technique on both benign [osteoid osteomas (OO) 1, 2, 3] and malignant bone tumors (metastases 3, 4, 5, 6, 7, 8).
The aim of this study is not only to assess the effectiveness of this treatment, but also to conduct a cost-benefit analysis as well as to consider how this treatment can be effective and curative if compared to other techniques more widely used particularly in the treatment of malignant tumours.
Focused ultrasound is based on destruction of the tissue through heat (thermoablation). By increasing the temperature of the tissue to a minimum level of 57° for a timeframe of about one second, protein denaturation is achieved and a coagulation necrosis is generated. Of outmost importance for the success of the treatment is that the ultrasound beam would appropriately reach the target, having the sufficient energy to cause a coagulation necrosis. To accomplish this goal it is necessary that:
- Patients are accurately recruited on the basis of the lesion site;
- Treatment is properly planned;
- Patients are accurately prepared for treatment.
As to the first point, accessibility to the lesion is important. It is known that the ultrasound penetration is hindered by bone, metallic devices, air, scars, or other obstacles causing aberration of the ultrasound beam. This is particularly relevant in the case of bone lesions, due to the acoustic attenuation in bone, which is ~30-60 times higher than in soft tissue. Hence, the lesions that benefit from HIFU are the more superficial ones where the ultrasound beam is almost completely absorbed by the bone, and only a low amount of energy is needed in order to achieve the desired clinical outcome. In our experience, it was difficult to treat lesions located deeper than 12 cm from the skin surface.
When the distance from skin surface to target area exceeds 10 cm due to interposition of soft tissues (muscles and muscular beams), it is possible that the ultrasound beam lose its effectiveness. Newly developed variants in the software (wave frequency, lesion depth, size and definition of the target area) may help to reach deeper tissues; nevertheless, there still exist intrinsic limitations of the technique that make its use not recommendable for some types of lesions. As an example, the system is designed to reduce frequency and optimize penetration of the beam. This, however, will result in a widening of the target area with subsequent lack of precision.
As to the second point, similarly to other techniques employed in interventional radiology, the treatment must be properly planned in order to avoid involvement and damage of other sensitive structures (intestine, nervous beams, minimally vascularized structures such as tendons and ligaments, that scarcely dissipate heat).
Last but not least, the patients must be accurately prepared to avoid interposition between skin surface and transducer. The patient skin must be accurately shaved. To adapt the transducer to the skin surface there are special components (water bags, gel pads, etc.) provided by the manufacturer. Each space must be filled with degassed water and US gel. Possible interposition of microbubbles between the skin surface and the transducer must be eliminated. Another aspect of capital importance is patient’s positioning and immobilization. The operators, in fact, must have the possibility to see all images, acquired after patient positioning, for planning and intra-procedural control. This is possible thanks to the employment of special supports (mattresses and cushions). Anesthesia plays an important role to prevent pain during the procedure. Through the continuous acquisition of MR images, it is possible to stop the delivery of energy, should any movement of the patient occurs. The optimal contrast resolution of MRI allows to accurately plan the treatment involving the entire lesion as well as to detect the presence of sensitive adjacent structures and avoid their damage. An additional advantage is represented by the possibility to combine contemporary imaging throughout the treatment of the lesion (with the use of specific sequences and contrast media).
Thermometry is enabled through the application of specific sequences [proton resonance frequency (PRF)] that allows real-time control of the reached temperature within the target area, by detecting all temperature-dependent MR phase variables. The images acquired during the treatment (when the tissue temperature increases progressively) are subtracted from those acquired prior to it. In this way, it is possible to follow the temperature pattern within the target area and to verify both the quantity of energy delivered and the presence of possible damage to the surrounding structures.
The use of thermoablation for the treatment of bone lesions has a double goal: palliation and size reduction. The first is accomplished through thermoablation at the periosteal level, an area that is richly innervated (this procedure has been applied also to benign lesions) (1,2). As to size reduction, it results from the direct thermoablation of both benign and malignant tissues.
Current employment in the musculoskeletal (MSK) field
Osteoid osteomas (OO)
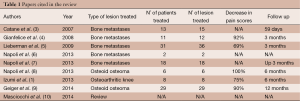
Patients with OO are those who benefit more from magnetic resonance guided focused ultrasound surgery (MRgFUS) that is currently becoming the technique of choice on the basis of a recent, though still limited, literature (Table 1) (8-10). Eighty percent of OOs are located superficially (at cortical or subendosteal level) and in the appendicular skeleton (50% at femoral and tibial level), representing this aspect an indication to the treatment. The appendicular skeleton, in fact, can be easily immobilized and is far from mobile or sensitive structures. The young age of the patients affected by OOs (mean age <25 years in 75% of patients) also represents an indication to HIFU that does not employ ionizing radiations, can be repeated and does not prevent the patients from being submitted to alternative techniques, in case of procedural failure, since their skin surface remains intact. For the clinical success of the treatment, therefore, and in consideration of the great variety of techniques available, the patients must be carefully recruited. The discriminating factor is represented by the presence of a proper acoustic window, which is defined as follows:
Full table
- Cortical location of the lesion which in any case must not be found deeper than 12 cm in the cortex;
- Absence of interfaces between skin surface and lesion (overall distance of max 10 cm);
- Absence of obstacles between skin and lesion (bone, metallic devices, etc.).
To treat the patients successfully with MRgFUS all these three requirements must be met. At present time, the option to treat lesions affecting the axial skeleton represents a crucial issue, due to the risk that the marrow be damaged by heat conduction through the vertebral bone.
Beside selection, also patient positioning plays an important role since it allows the US beam to optimally reach the target area. After patient positioning, the treatment duration can range from 20 seconds (the duration of one sonication) to some minutes, depending on the lesion size.
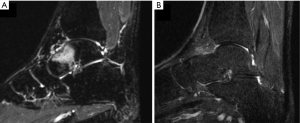
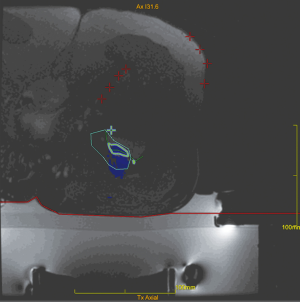
The operators receive a real-time feed-back of the treatment thanks to the thermal map which detects the areas where the temperature is high enough to generate a coagulation necrosis (Figure 1). After treatment, the patients do not refer functional disability and can go back to normal life the day after. At clinical level, even if MRgFUS is non-invasive, our results are similar to those obtained with radiofrequency (RF), as described also in other studies employing the same technique. Our experience started two years ago with the treatment of OOs. Out of 15 patients treated with MRgFUS as first choice, in 12 cases (80%) there was complete and immediate resolution of painful symptomatology soon after treatment, without recurrences or post-procedural complications. Post treatment VAS values after 2 years confirm the success of the procedure, ranging from 8.25 (pre-treatment) to 0.2 (post-treatment; 98% improvement in the VAS scale). As to the non-respondent patients (3 pts), it is worth mentioning that all of them (20%) were malrecruited, due to our scarce experience at those times. They presented a thick cortex (two cases) and a thick layer of subcutaneous tissues with multiple interfaces (one case). Retrospectively, it can be said that those patients should have been directly submitted to RF thermoablation. In that case, considering only the patients with the right indication (12 pts), a 100% success rate with MRgFUS technique would have been obtained. The therapeutical success, therefore, depends on the correct and accurate recruitment of the patients (Figure 2). The success of the treatment is also confirmed, with the MRI imaging, with the disappearance of the bone edema.
Benign epiphyseal lesions
The treatment of benign epiphyseal lesions (fibroangiomas, chondromas, etc.) (11,12) represents a challenge for the surgeon since the complexity of the intervention and the associated risks are not comparable to the biological aggressiveness of the lesion itself. The invalidating characteristics of these lesions, however, require a prompt intervention, which is made difficult by a variety of factors. They are of difficult access due to their location. Oftentimes, in fact, the surgeon opens more compartments and performs a demolitive intervention in order to guarantee the surgical sterility of the lesion, which often appears locally very aggressive, though being of low biological grade. In these cases, MRgFUS can be of great help thanks to its non-invasiveness and high clinical efficacy. It allows complete eradication of the lesion without damaging or impairing the surrounding structures. The absence of material devices (e.g., needles), used to transfer energy to the target area, excludes the risk of pathological cell dissemination. US beams do not leave any residual debris on the surrounding structures, allowing the possible submission to alternative surgical treatments. For an optimal therapeutic planning, orthopedic surgeon and interventional radiologist must make decisions in close cooperation about the diagnosis of the lesion, the option to perform a bioptic examination for pre-treatment disease confirmation, the presence of pathologic cell dissemination in other compartments, the possibility of a surgical treatment in case of procedural failure, and the presence of a proper acoustic window. A characteristic feature of the lesions treatable with thermoablation (not only with MRgFUS) is that they are surrounded by bone edema, as a sign of activity of the bone which tries to react to the lesion, causing pain. In these cases, thermoablation produces a necrosis of the active cells, allowing disappearance of edema and relief from pain.
At present time, there is no literature about the employment of MRgFUS in this field. In our Radiological Department a preliminary experience was carried out on 12 lesions (2 periosteal chondromas, 1 fibroangioma, 6 fibro-osteites, 2 fibromixo angiomas, 1 osteoblastoma), treated by means of MRgFUS in a 18-month period of time (our experience of non-invasive treatment of epiphyseal benign bone lesions using MRgFUS: can it really be a definitive solution? Arrigoni et al.—ECR 2014 March 6-10, Vienna, Scientific Paper B-0263) (Figure 3). All patients responded to therapy showing a marked improvement of painful symptomatology (mean VAS improvement at 12 months: 90%; pre-procedural VAS: 7.79; post-procedural VAS: 0.83). These quite satisfying clinical results were associated to good MRI and CT findings at the 12-month follow-up:
- No sign of lesion progression;
- In all cases, disappearance of reactive edema surrounding the lesion prior to treatment;
- Disappearance of the post contrast medium enhancement, when present;
- Initial lesion calcifications, as sign of loss of biological activity within the lesion itself.
In conclusion, though still in the preliminary phase, our experience shows that the employment of MRgFUS in this field is feasible and efficient.
Malignant lesions
Primitive malignant lesions are treated by the orthopedic surgeon and between the non-surgical treatment, the radiation therapy (RT) is the gold-standard choice. The interventional radiologist is demanded for palliation of secondary painful lesions by means of MRgFUS, or similar techniques (10,13-16). Moving from the assumption that only the lesions reachable by the US beam can be treated, thermoablation has the advantage to produce an immediate and complete necrosis of the cells where 57 °C can be reached for at least one second. Ionizing radiations employed in RT, on the contrary, kill the cells depending on the dose and on the cell division. Moreover, unlike RT, MRgFUS can be repeated: the repetition of a treatment with the RT is often impossible because of the cumulative toxicity of radiation to the healthy tissue that surrounds the tumor mass. MRgFUS is able to “sterilize” the lesions easily reached by the US beams, thanks to the possibility to monitor the temperature reached within the target area. This technique is particularly useful in the treatment of superficial lesions, originating from both bone (osteolytic lesions, with cortical erosion and extending beyond the bone cortex-extra-compartmental tumours) and soft tissues, abutting on the bone (with presence or not of a cleavage plane). These lesions benefit from MRgFUS technique because they absorb the US beam up to 30-60 times more than soft tissue. This leads to a marked increase of the temperature with subsequent necrosis of the superficial bone and adjacent tissues. The satisfactory bone capability to absorb US beams is at the basis of palliation of painful bone lesions. High energy delivered on the periosteum causes a thermal denervation of the periosteum itself with subsequent pain relief. Some authors in literature say that palliation is not only caused by this mechanism, but also by necrosis of tumoral tissue, which reduces the mass effect and leads to pain reduction. The main limitation, however, still remains the possibility to reach the lesion with the US beam. Aggressiveness of these lesions, in fact, requires the procedure to be as complete and accurate as possible. Comparative evaluations are necessary to make decisions about the most appropriate technique to use, also on the basis of a cost-benefit analysis. To date however, all the techniques of interventional radiology can be considered useful only for pain control and cannot be considered to tumor control (17,18).
Malignant lesions are difficult to be treated also due to the low survival rate of patients.
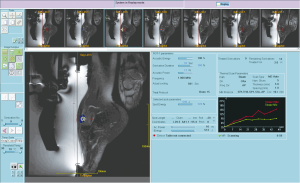
In our experience, six secondary bone lesions were treated for pain palliation (Figure 4). Mean time of the follow-up was 6 months and showed a 76% pain relief on the VAS scale. Each case was treated only once.
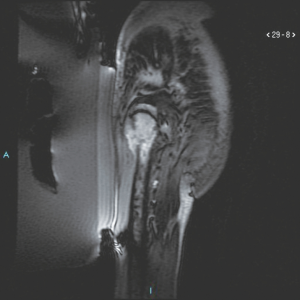
Of particular interest was the treatment of a lesion secondary to a lung neuroendocrine tumour, located at the level of the soft tissues of the thigh, adjacent to the femoral diaphysis. In this case, MRgFUS was employed in combination with surgery for thermoablation of the interface between femur and soft tissues, in order to facilitate the excision of the lesion by the surgeon. In this way it was possible by MRgFUS to “sterilize” the region between healthy and diseased tissues to avoid dissemination of the disease.
Conclusions
HIFUs represent nowadays a valid option in the treatment of both benign and malignant tumours. However, while waiting for studies carried out on larger series and longer term follow-ups, and considering the availability of alternative techniques within the field of interventional radiology, it is wise to employ MRgFUS strictly in those cases where this technique can be used as first choice, which is when the lesion can be easily reached by the US beam. Otherwise, the risk to leave the lesion only partially treated can turn into a total failure of the whole procedure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giusi Irma Forte and Giorgio Russo) for the series “High intensity focused ultrasounds” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.10.04). The series “High intensity focused ultrasounds” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Izumi M, Ikeuchi M, Kawasaki M, et al. MR-guided focused ultrasound for the novel and innovative management of osteoarthritic knee pain. BMC Musculoskelet Disord 2013;14:267. [PubMed]
- Weeks EM, Platt MW, Gedroyc W. MRI-guided focused ultrasound (MRgFUS) to treat facet joint osteoarthritis low back pain--case series of an innovative new technique. Eur Radiol 2012;22:2822-35. [PubMed]
- Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases--preliminary clinical experience. Ann Oncol 2007;18:163-7. [PubMed]
- Gianfelice D, Gupta C, Kucharczyk W, et al. Palliative treatment of painful bone metastases with MR imaging--guided focused ultrasound. Radiology 2008;249:355-63. [PubMed]
- Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol 2009;16:140-6. [PubMed]
- Napoli A, Anzidei M, Marincola BC, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 2013;33:1555-68. [PubMed]
- Napoli A, Anzidei M, Marincola BC, et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol 2013;48:351-8. [PubMed]
- Napoli A, Mastantuono M, Cavallo Marincola B, et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 2013;267:514-21. [PubMed]
- Geiger D, Napoli A, Conchiglia A, et al. MR-guided focused ultrasound (MRgFUS) ablation for the treatment of nonspinal osteoid osteoma: a prospective multicenter evaluation. J Bone Joint Surg Am 2014;96:743-51. [PubMed]
- Masciocchi C, Conchiglia A, Gregori LM, et al. Critical role of HIFU in musculoskeletal interventions. Radiol Med 2014;119:470-5. [PubMed]
- Thawait SK, Thawait GK, Frassica FJ, et al. A systematic approach to magnetic resonance imaging evaluation of epiphyseal lesions. Magn Reson Imaging 2013;31:418-31. [PubMed]
- Mellado JM, Bencardino JT, del Palomar LP. Magnetic resonance imaging features of the discrete epiphyseal radiolucency: a problem-solving approach to differential diagnosis. Curr Probl Diagn Radiol 2008;37:243-61. [PubMed]
- Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol 2004;22:300-6. [PubMed]
- Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423-36. [PubMed]
- Janjan N, Lutz ST, Bedwinek JM, et al. Therapeutic guidelines for the treatment of bone metastasis: a report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. J Palliat Med 2009;12:417-26. [PubMed]
- Dupuy DE, Liu D, Hartfeil D, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer 2010;116:989-97. [PubMed]
- Alongi F, Russo G, Spinelli A, et al. Can magnetic resonance image-guided focused ultrasound surgery replace local oncology treatments? A review. Tumori 2011;97:259-64. [PubMed]
- Orsi F, Arnone P, Chen W, et al. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther 2010;6:414-20. [PubMed]