
Gamma knife: a useful tool for treatment of essential tremor
Essential tremor (ET) is the most common movement disorder in the world. In the general population, the estimated prevalence is one percent (1) among adults aged 65 years and older, prevalence is approximately five percent (1). ET has a strong hereditary component, with approximately two-thirds of patients able to identify a relative with ET. Many patients with ET often share well-worn memories of parents, grandparents, aunts, and uncles severely affected by tremor. The pattern of inheritance is autosomal dominant with variable penetrance—over a lifetime the chance of a child of a person with ET developing tremor is one-half, though severity can vary. Incidence among men and women is about the same. Tremor typically occurs in the 5th or 6th decade of life, but may present as early as the teen years or much later in life.
While many may refer to ET as a “benign” condition, it is increasingly recognized as a serious slowly progressive neurological disorder (2,3). The tremor typically worsens with age, and can lead to severe difficulties with basic activities of daily living, such as eating, drinking, writing, opening or closing clothing buttons, or pressing buttons on a telephone or keyboard (4,5).
ET is characterized by an intention tremor of the arms or hands that occurs during voluntary movements, such as manipulating a cup or glass or using a pen or pencil to write. Tremor may also have an axial component, involving the head, neck, jaw, and voice. A minority of patients have axial tremor alone. Patients with ET may also have rest or postural tremor of the extremities, though this is not a hallmark of the disease.
Patients with ET experience a strong psychological component to their disease. Patients are often embarrassed in regard to their inability to enjoy and partake in basic aspects of work and social events such sharing a drink or meal (6). ET can lead to difficulties at work and employability across the spectrum of manual to intellectual labor—ET can equally hamper one’s ability to maneuver a drill or screwdriver as equally as a computer mouse or telephone keypad. As a result, tremor is disabling and can lead to significant depression and social phobia (7-9). The tremor is exacerbated by stress, and compounds social challenges for these patients.
ET is also poorly understood by many in the general population. Many do not understand the nature of this tremor, and the dampening effect of alcoholic beverages may lead to confusion around the relationship of an individual’s tremor. Those who suffer from severe ET may find that a few cocktails, glasses of wine, or beer before dinner render a much steadier hand for guiding a fork or knife. ET symptoms may paradoxically appear to worsen when one does not drink alcohol, sometimes leading to confusion that the tremor may be a sign of alcohol withdrawal.
The underlying cause of ET is unknown, although a great deal of insight into pathophysiological mechanism has been established (10,11). Studies suggest a tremor generator in the inferior olivary nucleus. The tremor signal is then transmitted through cerebellar tracts to the thalamocortical motor circuits. Electrical recordings from the ventral intermediate nucleus (VIM) of the thalamus demonstrate neurons that discharge excessively and synchronously with the tremor.
First line medical therapy for ET is treatment with an anti-epileptic drug or beta-blocker. The two most commonly prescribed medications are primidone and propranolol, though up to one-half of patients will not respond to either drug (12). Studies assessing up to one year of treatment with these two medications show improvement of tremor. However, multiple studies demonstrate a loss of effectiveness over time, need for increasing doses to control tremor, and side effects associated with these medications (13-16).
For patients with severe medically refractory tremor, radiofrequency thalamotomy of the VIM was the surgical procedure of choice for many years, but more recently deep brain stimulation (DBS) has replaced open surgical thalamotomy. Proponents of DBS for the surgical treatment of ET advocate the nondestructive and reversible nature of the procedure, and its effectiveness in relieving ET. However, DBS is an invasive procedure not without risks. Significant side effects of DBS acutely include intracerebral hemorrhage or ischemic infarction associated with hemiparesis and/or decreased consciousness. Potential hardware complications in the long term include discomfort, wound infections, lead malposition and/or migration, component fracture, component malfunction, skin erosion, cognitive decline, and loss of effect (17-24). Further, patients with implanted DBS hardware may be at risk for serious and even life threatening injuries from diathermy, MRI imaging studies performed with a body coil, or electrical currents generated by surgical electrocautery equipment (25-29). Anesthesia risks in the elderly also are significant.
In an effort to avoid these complications and provide definitive therapy for patients who are not also candidates for DBS, multiple retrospective series have been published examining Gamma knife thalamotomy for ET.
Gamma knife radiosurgery is a non-invasive stereotactic radiosurgical procedure with a decades-long track of successful treatment of intracranial neoplasms and functional disorders. Patients are immobilized in an aluminum headframe and undergo axial imaging of the brain, which is reconstructed in three dimensions for precise localization of treatment targets with reference to the fixed headframe coordinate system. A radiation dose in the range of 130-140 Gy is prescribed to the target 100% isodose point within the thalamic VIM.
Multiple centers have published recent results on use of gamma knife radiosurgery treatment for ET (30-33). Three of the larger studies have shown effectiveness in the range of 80% (30-32). A smaller study from France with blinded assessments demonstrated improvement by 55%. Permanent side effects related to Gamma knife radiosurgery are in the range of zero to four percent in these studies. In the Pitttsuburgh series reported by Kooshkabadi et al. (86 patients), two patients developed a temporary contralateral hemiparesis, one patient noted dysphagia, and one patient developed sustained facial sensory loss. In the series from Seattle reported by Young et al. (161 patients), 14 patients suffered neurological side effects—six were temporary and 8 were permanent (two patients had permanent sensory deficits of no function consequence, others had difficulty with speech or contralateral motor function).
Across series, most patients experience tremor control in two to three months. Serial writing and drawing scores (and other measures, e.g., drinking water from a cup) are obtained at baseline and in follow-up. Tremor scores are most commonly assessed according to the rating scale of Fahn, Tolosa, Marin (34).
A patient may be considered for a contralateral Gamma knife treatment if a good response to treatment is obtained initially, though a 12 month waiting period is necessary to be sure patients are outside of the reported side effect period. A patient’s pre- and post-treatment drawing samples are shown in Figure 1. Post-treatment effect characterized by MR imaging in a patient undergoing contralateral Gamma knife thalamotomy is shown in Figure 2.
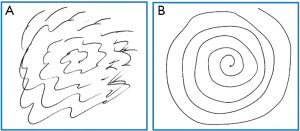
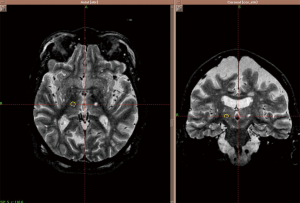
Overall, ET is a serious challenge for many individuals. Gamma knife thalamotomy is a safe and effective noninvasive approach to treatment of ET, worthy of consideration in many patients. It is especially relevant as a treatment option for medically refractory tremor in the elderly or those with contraindications to DBS.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sandra Vermuelen, Kevin T. Murphy, Huan Giap) for the series “SBRT/SRS in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.08.01). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534-41. [PubMed]
- Deuschl G, Elble R. Essential tremor--neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord 2009;24:2033-41. [PubMed]
- Deuschl G, Raethjen J, Hellriegel H, et al. Treatment of patients with essential tremor. Lancet Neurol 2011;10:148-61. [PubMed]
- Koller W, Biary N, Cone S. Disability in essential tremor: effect of treatment. Neurology 1986;36:1001-4. [PubMed]
- Bain PG, Findley LJ, Atchison P, et al. Assessing tremor severity. J Neurol Neurosurg Psychiatry 1993;56:868-73. [PubMed]
- Lorenz D, Schwieger D, Moises H, et al. Quality of life and personality in essential tremor patients. Mov Disord 2006;21:1114-8. [PubMed]
- Woods SP, Scott JC, Fields JA, et al. Executive dysfunction and neuropsychiatric symptoms predict lower health status in essential tremor. Cogn Behav Neurol 2008;21:28-33. [PubMed]
- Schneier FR, Barnes LF, Albert SM, et al. Characteristics of social phobia among persons with essential tremor. J Clin Psychiatry 2001;62:367-72. [PubMed]
- Louis ED, Barnes L, Albert SM, et al. Correlates of functional disability in essential tremor. Mov Disord 2001;16:914-20. [PubMed]
- Deuschl G, Elble RJ. The pathophysiology of essential tremor. Neurology 2000;54:S14-20. [PubMed]
- Elble RJ. Central mechanisms of tremor. J Clin Neurophysiol 1996;13:133-44. [PubMed]
- Koller WC, Vetere-Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 1989;39:1587-8. [PubMed]
- Calzetti S, Sasso E, Baratti M, et al. Clinical and computer-based assessment of long-term therapeutic efficacy of propranolol in essential tremor. Acta Neurol Scand 1990;81:392-6. [PubMed]
- Gorman WP, Cooper R, Pocock P, et al. A comparison of primidone, propranolol, and placebo in essential tremor, using quantitative analysis. J Neurol Neurosurg Psychiatry 1986;49:64-8. [PubMed]
- Sasso E, Perucca E, Fava R, et al. Primidone in the long-term treatment of essential tremor: a prospective study with computerized quantitative analysis. Clin Neuropharmacol 1990;13:67-76. [PubMed]
- Serrano-Dueñas M. Use of primidone in low doses (250 mg/day) versus high doses (750 mg/day) in the management of essential tremor. Double-blind comparative study with one-year follow-up. Parkinsonism Relat Disord 2003;10:29-33. [PubMed]
- Appleby BS, Duggan PS, Regenberg A, et al. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years’ experience. Mov Disord 2007;22:1722-8. [PubMed]
- Beric A, Kelly PJ, Rezai A, et al. Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg 2001;77:73-8. [PubMed]
- Binder DK, Rau G, Starr PA. Hemorrhagic complications of microelectrode-guided deep brain stimulation. Stereotact Funct Neurosurg 2003;80:28-31. [PubMed]
- Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: a review of the published literature. Stereotact Funct Neurosurg 2006;84:248-51. [PubMed]
- Kenney C, Simpson R, Hunter C, et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg 2007;106:621-5. [PubMed]
- Koller WC, Lyons KE, Wilkinson SB, et al. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord 2001;16:464-8. [PubMed]
- Woods SP, Fields JA, Lyons KE, et al. Pulse width is associated with cognitive decline after thalamic stimulation for essential tremor. Parkinsonism Relat Disord 2003;9:295-300. [PubMed]
- Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg 2014;120:132-9. [PubMed]
- Henderson JM, Tkach J, Phillips M, et al. Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson’s disease: case report. Neurosurgery 2005;57:E1063; discussion E1063.
- Rezai AR, Baker KB, Tkach JA, et al. Is magnetic resonance imaging safe for patients with neurostimulation systems used for deep brain stimulation? Neurosurgery 2005;57:1056-62; discussion 1056-62. [PubMed]
- Roark C, Whicher S, Abosch A. Reversible neurological symptoms caused by diathermy in a patient with deep brain stimulators: case report. Neurosurgery 2008;62:E256; discussion E256.
- Sharan A, Rezai AR, Nyenhuis JA, et al. MR safety in patients with implanted deep brain stimulation systems (DBS). Acta Neurochir Suppl 2003;87:141-5. [PubMed]
- Spiegel J, Fuss G, Backens M, et al. Transient dystonia following magnetic resonance imaging in a patient with deep brain stimulation electrodes for the treatment of Parkinson disease. Case report. J Neurosurg 2003;99:772-4. [PubMed]
- Young RF, Li F, Vermeulen S, et al. Gamma Knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg 2010;112:1311-7. [PubMed]
- Kooshkabadi A, Lunsford LD, Tonetti D, et al. Gamma Knife thalamotomy for tremor in the magnetic resonance imaging era. J Neurosurg 2013;118:713-8. [PubMed]
- Ohye C, Higuchi Y, Shibazaki T, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. Neurosurgery 2012;70:526-35; discussion 535-6. [PubMed]
- Régis J, Carron R, Azulay JP, et al. Gamma knife radiosurgery thalamotomy for intractable tremors: a blinded assessment. Journal of Radiosurgery & SBRT 2013;2:171.
- Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E. eds. Parkinson’s Disease and Movement Disorders, 2nd ed. Baltimore: William & Wilkins, 1993:271-80.


