
Molecular mechanisms involved in tumor repopulation after radiotherapy
Introduction
Ionizing radiation was used as a means to treat cancer soon after Wilhelm Roentgen discovered X-rays. Modern radiation therapy was based on a fractionated scheme instead of a single high dose of radiation. The fractionated dose scheme was based on the well known ram’s testes experiments in 1927 by Regaud and Ferroux done in France (1). In their experiments, when a ram’s testes were irradiated for sterilization, a single dose exposure failed to sterilize the ram despite severe scrotum skin injury. On the other hand, a fractionated dose scheme was successful in sterilizing the ram. This fundamental discovery were gradually adopted in the filed of radiation oncology worldwide in the form of fractionated radiotherapy which is still the norm in radiation oncology. At the theoretical level, fractionated radiotherapy was based on the theory of the 4“R”s (2) (repair, redistribution, reoxygenation and repopulation) which are described briefly here.
Repair is correlated with the cell’s ability to form DNA strand breaks. Treatment to use fractionated doses (usually 1.8-2.0 Gy/day) with a time interval (0.5-24 hrs depending on cell types) will allow cells to recover from most of the sublethal damage after the irradiation. It has been assumed that normal healthy cells will be able to activate their checkpoint mechanisms and repair the “sublethal” damage. On the other hand, most types of cancer cells have deficiencies in their checkpoint mechanisms and thus less able to repair DNA damage. Therefore multiple fractions of radiation allow normal cells to carry out repair while allowing tumor cells to be exposed to higher level of radiation. Redistribution refers to radiation-induced cell cycle effects. Because cancer cells are more sensitive in G2/M phases of the cell cycle than G1/S and they tend to pile up in G2/M due to a functional G2 checkpoint after being exposed to radiation, they are more likely to be killed during subsequent irradiation. In comparison, normal cells are mostly in G0/G1 due to a G1 checkpoint and are thus less susceptible to this type of sensitization (3). Re-oxygenation refers to the changes in oxygen tension within the irradiated tumor mass. In low LET photon radiotherapy, oxygen molecule is key for radiation induced cell killing because it facilitates the formation of free radical species that are responsible for most of radiation induced DNA damage (4). Hypoxic tumor cells are thus much more difficult to kill than well oxygenated ones. If radiation treatment is fractionated, the hypoxic cells will be allowed to reoxygenate due to reduced demand from dying tumor cells and the subsequent fractions will be much more efficient to eliminate the reoxygenated tumor cells. Repopulation is the rapid proliferation of surviving tumor cells after radiation induced cell killing (5). The influence of repopulation on the outcome of radiotherapy is self-evident. Effective suppression of tumor cell repopulation is therefore key for the success of radiotherapy.
In this review, we summarize some of the key recent discoveries that have added significantly to our knowledge base of tumor response to radiotherapy. We hope the discussion can stimulate fresh new endeavors into this important area of cancer research.
The importance of tumor vasculature vs. tumor cells in radiotherapy
One notable recent controversy in molecular radiation biology is the relative importance of tumor cells vs. tumor vasculature. Most of 4“R”s are mainly centered on how to sensitize tumor cells to radiation. In a study published in 2003 (6), Kolesnick and colleagues demonstrated that tumor vasculature could play a key role. Using a transgenic mouse model that was rendered resistant to apoptosis induction in the endothelial cell compartment due to knockout (KO) of the asmase or Bax genes, the authors demonstrated that tumors were significantly more resistant to radiation when their vasculature was rendered more resistant to apoptosis. In addition, they showed that when a higher dose of radiation was used to kill the endothelial cells in the KO mice, tumors would be effectively controlled. This study caused controversy because it challenged established, tumor cell-centric concepts in radiobiology. The data were also quite different from an earlier study (7) that showed the tumor control dose (TCD50) in a radiation sensitive mouse (SCID) background was not significantly different from that in a non-sensitive (nude) background. In that same study, however, it was shown that stroma sensitivity to radiation did cause significant tumor growth delay. In a more recent study, Gerweck et al. (8) showed that tumor cells that were deficient in the DNA-PKcs gene and thus very sensitive to radiation, showed significantly less growth delay after irradiation when compared with its genetically identical counterpart with the DNA-PKcs gene. The results were interpreted as indicating that tumor cell sensitivity did matter for overall tumor response to radiotherapy. In a further paper combining genetically identical tumor cells lines with or without DNA-PKcs and host mice with or without DNA-PKcs deficiency (9), it was shown that radiation sensitivities of both tumor cells and stromal tissues play important roles in determining the outcome of radiotherapy.
Importance of bone marrow derived cells in tumor response to radiotherapy
Since Garcia-Barros et al. (6) demonstrated the importance of tumor endothelial cells in determining the outcome of radiotherapy, other studies have shown that additional non-tumor cells also play significant roles. For example, Ahn and colleagues have shown that vasculogenesis, the de novo formation of blood vessels, to be important in tumor recovery. They showed a crucial role for matrix metalloproteinase-9 (MMP-9) in mediating tumor vasculogenesis (10). MMP-9 is a protein involved in extracellular matrix degradation and a member of zinc-containing endopeptidases (11). In a MMP-9 KO mouse model, tumor growth were completely inhibited in pre-irradiated hosts but restored after wild-type bone marrow cells were transplanted into the MMP-9 KO mice (10). Surprisingly, they found that BM-derived CD11b+ myelomonocytic cells were the most recruited to X-irradiated tumor for vasculogenesis rather than epithelial progenitor cells, which had previously been shown to be important for tumor blood vessel development (12). Other studies have shown that tumors recruited myeloid cells via secretion of VEGF (13) and M-CSF (14) through VEGF receptor-1 (15) and M-CSF receptor (16) respectively to activate their migration to the tumor. Subsequently, myeloid cells might produce proangiogenic cytokine, including stromal derived factor-1 (SDF-1), VEGF, TGF-β. Of note is an additional study by Kioi et al. (17) which demonstrated in a mouse glioma model that radiation activated HIF-1 which stimulated the transcription of SDF-1 that caused the homing in of bone marrow derived CD11b+ myelomonocytes to induce vasculogenesis. A small molecule drug AMD3100 appears to be effective in suppressing tumor growth when used in combination with radiotherapy.
HIF-1 as a major regulator of tumor response to hypoxia and radiotherapy
Hypoxia, a condition of oxygen tension below the physiological norm, has long been recognized as a common feature of the tumor microenvironment. Hypoxia in itself can significantly increase radiation resistance of tumor cells due to its ability to reduce radiation induced free radicals, which are the main effectors in radiation induced cell killing. In addition, at the biological level, hypoxia induces profound changes in tumor cells that allow it to be more angiogenic and metastatic (18-20). Previously, it has been identified that HIF-1 transcription factor is the master regulator that coordinate cellular response to hypoxia (21,22). A rich body of literature has established HIF-1α as the key factor that plays a central role in tumor angiogenesis and tumor proliferation under hypoxic conditions. Under normoxic conditions, HIF-1α is hydroxylated by proline hydroxylases (PhDs) in an oxygen-dependent manner. The hydroxylation key proline residues in HIF1α leads to rapid recognition by VHL and subsequent ubiquitylation of HIF-1α, which leads to proteasome-mediated degradation (23). Under hypoxic conductions, HIF-1α is not hydroxylated and the protein remains stabilized and able to activate downstream genes. In addition to the oxygen-dependent activation, studies have shown that HIF-α could be activated in a hypoxia-independent manner by radiotherapy (24). It was shown that irradiation of tumor cells could result in increased nuclear accumuation and enhanced translation of HIF-1α after radiation induced depolymerizaton of “stress granules” (24). In another study, Li et al. showed that after radiotherapy, tumor associated macropahges mediate hypoxia independent activation of HIF-1α through a nitric oxide mediated mechanism (25). They showed that L-NAME, a potent inhibitor of NO synthases (NOS), can attenuate HIF-1α activity in 4T1 murine breast tumors, which suggested that, NOS was likely to be the source of NO in enabling radiation induced HIF-1α stabilization. They further identified that iNOS (inducible NOS) in the tumor-associated macrophages (TAMs) was the source of NO production after radiotherapy (Figure 1). NO was shown to nitrosylate the Cys533 residue in the HIF-α oxygen-dependent domain in mouse cells (correspond to Cys520 in human HIF-1α). Nitrosylation of HIF-1α at Cys533 protected HIF-1α from degradation by preventing its binding to von Hippel-Lindau (vHL). The discovery of an NO-based HIF-1α activation mechanism in response to radiation has opened up an option to use NOS inhibitor to attenuate tumor HIF-1α activation and suppress tumor growth (25).

The unexpected roles of caspase 3 in tumor cell repopulation after radiotherapy
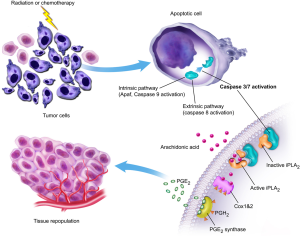
Tumor repopulation is an important mechanism through which tumors growth back after radiotherapy (2). Despite the recognition of its importance for decades, the mechanism for repopulation, especially accelerated tumor repopulation (5) in some cancer patients undergoing radiotherapy, is not clear. A recent study from our group shows that one of the key signals for tumor repopulation after radiotherapy is actually cell death induced by radiation (26). We show that lethally irradiated tumor or fibroblast cells can stimulate the rapid proliferation of non-irradited tumor cells in tissue culture or in mice. In addition, we show that caspase 3 activation in the dying cells is key for the growth-stimulating signals. In casp3-/- MEF cells, the growth-stimulation effect is significantly attenuated. Given that caspase 3 itself is considered a cellular “excutioner” whose normal function is to get rid of damaged or unwanted cells, its positive regulation of a signal that stimuates tumor cell repopulation is especially surprising. Further experiments show that one of the major downstream factors that regulate cell growth is calcium-independent phospholipase A2 (iPLA2), which is cleaved and activated by caspase 3. Caspase 3-mediated activataion of iPLA2 leads to increased production of arachidonic acid, which in turn boosts the production of PGE2 that stimulates tumor growth (Figure 2). We named this newly discovered tumor cell repopulation mechanism the “phoenix rising” pathway. In a separate study, we show that the “phoenix rising” pathway is a fundamental mechanism for wound healing and tissue regeneration (27). Our discovery in the normal tissue is consistent with earlier discoveries in lower organisms that were characterized as “compensatory proliferation” (28,29).
Involvement of cancer stem cells in tumor response to radiation therapy
One of the major new concepts emerging in the past decade is cancer stem cells. Cancer stem cells were initially described by John Dick and colleagues in human acute myeloid leukemia (30). At earlier times cancer cells in a tumor mass were largely treated as clonal and mostly identical, except for rare mutants. The discovery of cancer stem cells completely changed this viewpoint. Cancer stem cells rapidly become a focal point of attention because they are the putative cells responsible for tumor cell self-renewal. Targeting cancer cells would be akin to eradicating the roots of the tree. Eliminating of cancer stem cells alone may be sufficient to suppress the growth of the whole tumor. Earlier studies do show that human cancer stem cells possess remarkable ability to form tumors in nude mice. For example, it was shown that as few as 100 CD44+CD24- breast cancer stem cells could form tumors in a nude mouse (31). In contrast 105 non-stem cells could not form tumors in the same mice. Another important characteristic of cancer stem cells appears to be their resistance to chemotherapy and radiation therapy. It was shown in animal models that cytoxic treatment of cancers increased the percentage of cancer stem cells, indicating their relative resistance to these agents. At the mechanistic level, it was shown that glioma stem cells had the ability to upregulate their DNA repair capacity to deal with DNA damage inflicted on them by exposure to radiation (32). Similer radiation-resistant properties of cancer stem cells were reported in breast cancer cells. By use of colony forming assay, it was shown that cancer cells bearing stem cell markers were significantly more radio-resistant than those cells without the markers (33). These appeared to provide strong rationale for developing strategies to target cancer stem cells during conventional chemotherapy or radiation therapy.
Counter-arguments against sole targeting of cancer stem cells during cancer therapy
Despite signicant enthusiasms among the cancer research community towards cancer stem cells as key targets in cancer therapy, there are also increasing evidence that there are complicated biology and confusion that need to be sorted out. One area that has generated a lot of controveries is the assay system for the “stemness” of cancer stem cells. Currently the “gold standard” is the ability to form tumors in immunodeficient mice. However, Quintana et al. show that the use of different mouse strains may lead to drastically different estimation of the frequencies of cancer stem cell in patient-derived melanoma samples. For example the use of NOD/SCID mice, which of the host of choice for estimating the frequencies of cancer stem cells in patient tumor samples, often leads to an estimate of 1 in a million (0.00001%) human melanoma cells as tumorigenic. However, if the same samples were itradiated in NSD (NOD/SCID interleukin 2-receptor gamma chain null) mice, the frequency of stem cells can be as high as one in three (34). These data strongly suggest that previous estimates of cancer stem cell frequencies are very much subjected to the assay system. The other area of confusion is the markers used to define stem cells. Different groups have used different markers for the same type of tumor, most of them on cellular surface (e.g., ABCB5, CD166, CD271 for melanoma), a few based on intracellular enzyme staining (ALDH1, or side population). Therefore, there is no consensus on a set of markers that can be universally applied to isolate cancer stem cells from tumor samples. This led to many problems that include wildly different estimates of the frequency of cancer stem cells. It could also lead to problems in efforts to target cancer stem cells because of the lack of consensus cancer stem cell markers and mechanisms.
Epigenetic reprogramming, a further issue that complicates the cancer stem cell field
Much of the intial enthusiasm on cancer stem cells is based on the initial assumption of a strict hierarchical structure in cancer cells in a tumor mass, similar to those found in normal tissues such as the hematopoietic system. However, there are several studies now indicating that the percieved hierarchy structure may not exist in cancer cells. For example, it was found that in melanoma tissues, the putative non-stem cell faction could form tumors equally as well as the stem cell faction. In addition, the newly formed tumors contain cancer cells that now possess the stem cell markers, indicating the plasticity of the cancer stem cell marker expression (35). Consistently, in another study it was found that ionzing radiation could induce the expression of stem cell genes such as Oct4, Sox2, Nanog, or Klf4 in breast cancer cells (36). This finding, in particular, calls for the re-examination of previous studies that reported the enrichment of cancer stem cells after treatment with conventional chemotherapeutic agents. It is possible that the observed increase in stem cells fraction may come from reprogramming of relatively differentiated cancer cells instead of expansion of pre-existing cancer stem cells. Indeed, other several other stimuli such as hypoxia condition (37) and nitric oxide-induced notch signaling (38) have been shown to induce epigenetic reprogramming in gliomablastoma cells. Interestingly, in a published study from our own laboratory, we observed that caspases 3&8 are activated by the transduction of the so-called Yamanada factors. Furthermore, we show that activation of the caspases facilitated the reprogramming of human fibroblasts into induced pluripotent stem cells instead of killing the cells (39). Therefore, it is conceivable that during cancer therapy induced caspase activation could faciliate cellular reprogramming if the cells somehow survive the caspase activation.
Conclusions
The classical 4“R”s have served the field of radiation cancer therapy very well. In the past two decades, we are beginning to understand the genetic, epigentic, and microenvironmental mechanisms underpining the 4“R”s. We hope the new insights gained will provide the basis for the development novel therapeutic agents and approaches that can significantly enhance current radiotherapy.
Acknowledgments
Funding: This work is supported by grants CA131408, CA136748, and CA155270 from the US National Institutes of Health (C-Y L).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Stem Cells in Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.10.03). The series “Stem Cells in Cancer” was commissioned by the editorial office without any funding or sponsorship. CYL served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Regaud C, Ferroux R. Disordance des effets de rayons X, d’une part dans le testicule, par le fractionnenment de la dose. Comptes Rendus Societe Biologique 1927;97:431.
- Withers HR. The 4 R’s of radiotherapy, in Advances in radiation biology. Lett JT, Alder H. eds. Vol 5. New York: Academic Press, 1975;5:241-71.
- Sinclair WK. Cyclic x-ray responses in mammalian cells in vitro. Radiat Res 1968;33:620-43. [PubMed]
- Hall EJ. Radiobiology for the radiologist. 4 ed. Philadelphia: Lippincott, 1994.
- Withers RH. Treatment-induced accelerated human tumor growth. Semin Radiat Oncol 1993;3:135-43. [PubMed]
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155-9. [PubMed]
- Budach W, Taghian A, Freeman J, et al. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst 1993;85:988-93. [PubMed]
- Gerweck LE, Vijayappa S, Kurimasa A, et al. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res 2006;66:8352-5. [PubMed]
- Ogawa K, Boucher Y, Kashiwagi S, et al. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res 2007;67:4016-21. [PubMed]
- Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008;13:193-205. [PubMed]
- Heissig B, Hattori K, Friedrich M, et al. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol 2003;10:136-41. [PubMed]
- Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 2001;7:1194-201. [PubMed]
- Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008;13:206-20. [PubMed]
- Kubota Y, Takubo K, Shimizu T, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 2009;206:1089-102. [PubMed]
- Beck H, Raab S, Copanaki E, et al. VEGFR-1 signaling regulates the homing of bone marrow-derived cells in a mouse stroke model. J Neuropathol Exp Neurol 2010;69:168-75. [PubMed]
- Ikeda O, Sekine Y, Muromoto R, et al. Enhanced c-Fms/M-CSF receptor signaling and wound-healing process in bone marrow-derived macrophages of signal-transducing adaptor protein-2 (STAP-2) deficient mice. Biol Pharm Bull 2008;31:1790-3. [PubMed]
- Kioi M, Vogel H, Schultz G, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010;120:694-705. [PubMed]
- Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer 1999;80:1697-707. [PubMed]
- Rofstad EK, Halsør EF. Hypoxia-associated spontaneous pulmonary metastasis in human melanoma xenografts: involvement of microvascular hot spots induced in hypoxic foci by interleukin 8. Br J Cancer 2002;86:301-8. [PubMed]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer 2002;2:38-47. [PubMed]
- Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 1995;92:5510-4. [PubMed]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A 1993;90:4304-8. [PubMed]
- Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468-72. [PubMed]
- Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004;5:429-41. [PubMed]
- Li F, Sonveaux P, Rabbani ZN, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 2007;26:63-74. [PubMed]
- Huang Q, Li F, Liu X, et al. Caspase 3-mediated stimulation of tumor cell repopulation during Cancer radiotherapy. Nat Med 2011;17:860-6. [PubMed]
- Li F, Huang Q, Chen J, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal 2010;3:ra13. [PubMed]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 2004;7:491-501. [PubMed]
- Chera S, Ghila L, Dobretz K, et al. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell 2009;17:279-89. [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast Cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [PubMed]
- Bao S, Wu Q, Mclendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756-60. [PubMed]
- Phillips TM, Mcbride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 2006;98:1777-85. [PubMed]
- Quintana E, Shackleton M, Sabel MS, et al. Efficient tumour formation by single human melanoma cells. Nature 2008;456:593-8. [PubMed]
- Quintana E, Shackleton M, Foster HR, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010;18:510-23. [PubMed]
- Lagadec C, Vlashi E, Della Donna L, et al. Radiation-induced reprogramming of breast Cancer cells. Stem Cells 2012;30:833-44. [PubMed]
- Heddleston JM, Li Z, Mclendon RE, et al. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a Cancer stem cell phenotype. Cell Cycle 2009;8:3274-84. [PubMed]
- Charles N, Ozawa T, Squatrito M, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 2010;6:141-52. [PubMed]
- Li F, He Z, Shen J, et al. Apoptotic caspases regulate induction of iPSCs from human fibroblasts. Cell Stem Cell 2010;7:508-20. [PubMed]


