
Use of yttrium-90 radioembolization for patients with unresectable liver metastases: experience from a single center
Introduction
Yttrium-90 (Y-90) microspheres radioembolization is an intra-arterial, catheter-based technique that delivers high doses of internal radiation to liver tumors. Y-90 is embedded into non-biodegradable microspheres which are selectively administered to branches of the hepatic artery. The goal of radioembolization is to deliver tumoricidal doses of radiation to tumors while sparing surrounding liver parenchyma. Radioembolization takes advantage of the unique vascular supply of the liver and hepatic solid tumors. Liver metastases are hypervascular lesions deriving 80–100% of their blood supply from the hepatic artery as compared with the liver parenchyma which relies on the portal vein (1,2). Microspheres embedded with Y-90 when installed through the hepatic artery concentrate in liver tumors in a 3:1 to 20:1 ratio when compared with normal liver parenchyma. As a result, liver tumors in particular are favorable targets for intra-arterial therapies based on this preferential blood supply.
Y-90 is a pure beta-emitter with a mean tissue penetration of 2.5 mm. A dose of 1 GBq contains approximately 25 million microspheres and provides about 50 Gy per kilogram. Y-90 microspheres have been found to deliver highly effective radiation doses (100 to 1,000+ Gy) to tumor tissue (3). Conventional external beam radiation is limited by the extreme radiosensitivity of liver parenchyma; whole liver external beam radiation is limited to 30 Gy to avoid radiation-induced liver toxicity (4). At present, two commercially available products are available, SIR-Spheres and TheraSpheres. SIR-Spheres were approved for colorectal cancer with metastasis to the liver in conjunction with continuous intrahepatic infusion floxuridine (FUDR).
The liver is the most common site of metastases in primary gastrointestinal malignancies because of hematogenous spread of malignant cells through the portal circulation. In addition, the presence of unresectable hepatic metastases is an independent sign of poor prognosis (5,6). The five year survival rate for patients with hepatic metastases ranges from 23–45% (7). Surgical resection is the treatment of choice for eligible patients as it may potentially result in clinical cure. Candidacy for surgical resection of liver metastases is generally based upon medical fitness for surgery, the functional reserve of the liver, the number and size of lesion lesions, lymph node/vascular involvement, and the presence of extra-hepatic disease, although criteria differ among individual surgeons and institutions (8,9).
Unfortunately, only 10% to 20% of metastatic colorectal carcinoma is resectable (10). Without intervention, colorectal liver metastases have a reduced median survival of 9 months (11). Cholangiocarcinoma and other biliary cancers are often diagnosed at an advanced stage, requiring resection of both primary and metastatic disease, which is associated with high operative mortality. For endocrine tumors with liver metastases, the 5-year survival for untreated disease is 13–54%, but only 10–20% of these cases are candidates for complete resection with curative resection often not possible (12). As a result, there has been continued interest in defining the role of alternative regional treatments for disease control including local tumor ablation, regional hepatic intra-arterial chemotherapy or chemoembolization, radiation therapy, and intra-arterial radioembolization.
To date the safety and efficacy of Y-90 radioembolization in the management of liver metastases has been promising. In a 2014 review of 20 studies (979 patients), patients with unresectable metastatic colorectal cancer who failed chemotherapy had a median survival of 12 months after Y-90 treatment, with 0% complete response (CR), 31% partial response (PR), and 40.5% stable disease (SD); median time to intrahepatic progression was 9 months (13). A prospective study by Rafi et al. treated 19 patients with cholangiocarcinoma with liver involvement, with 0% CR, 11% PR, 68% SD, and 21% progression of disease; median survival of 12 months after start of Y-90 (14). A prospective study by Cao et al. examined 51 patients with neuroendocrine metastases treated with radioembolization, with 12% CR, 27% PR, 27% SD, and 33% progression of disease; median survival was 36 months (15). However, a 2009 Cochrane review concluded that well-designed, large, phase III clinical trials were still needed to confirm the effectiveness of radioembolization of colorectal metastasis to the liver (16).
Methods
Patient cohort
Between 2005 and 2015, 21 patients with unresectable liver metastases were treated with Y-90 microspheres at Scripps Clinic. Eligibility for radioembolization was determined by treating providers involving a multi-disciplinary team of medical oncologists, radiation oncologists, interventional radiologists, and surgeons. Selection criteria included the following: the presence of unresectable liver dominant metastases, progressive disease (PD) despite systemic therapy, disease not amenable to alternative locoregional therapies, age greater than 18 years old, clinically acceptable pretreatment performance status, and ability to undergo pretreatment angiography. Exclusion criteria included uncorrectable blood flow to the gastrointestinal tract, significant extra-hepatic disease, and applied lung dose greater than 30 Gy in a single treatment fraction.
A comprehensive review of each patient’s medical record was performed including baseline patient characteristics, toxicities, radiographic imaging, and survival outcomes. Data were collected retrospectively. The study was approved by our institutional review board, and patient data were deidentified compliant with the Health Insurance Portability and Accountability Act. This work conforms to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000).
Pretreatment evaluation and staging
All patients included in this study were diagnosed with secondary metastatic liver cancer with tumor biopsy, radiographic imaging, or a combination thereof. Pretreatment evaluation included comprehensive history and physical, routine laboratory tests, and baseline imaging studies. Primary tumor staging was accomplished by American Joint Committee on Cancer (AJCC) TNM staging system.
Intervention
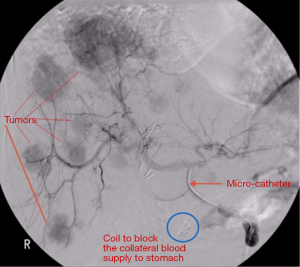
All patients underwent pretreatment computed tomography (CT) scan of the chest and abdomen in order to determine liver lobe volumes. Celiac and hepatic angiography was performed to define hepatic vascular anatomy. Patients with hepatic arteries supplying gallbladder, stomach, or intestine underwent coil embolization of collateral arteries or were otherwise excluded from therapy (Figure 1). A Tc-99-macro-aggregated albumin (MAA) was performed at the time of the planning angiogram to determine lung shunting for planning purposes to avoid complications of radiation pneumonitis (Figure 2).
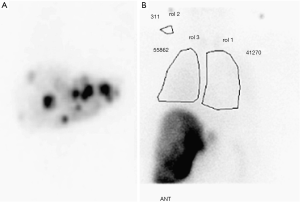
The prescribed activity of Y-90 for resin microsphere was determined according to body surface area (BSA) method outlined in the User’s Manual and Package Insert provided by the manufacturer. The method varies the prescribed activity based upon the size of the patient as well as the proportion of tumor involvement of the liver. The activity delivered was reduced if there was evidence of increased lung shunt. Microspheres activity was determined utilizing conventional Medical Internal Radiation Dose (MIRD) Committee technique adjusted according to the calculated shunt of lung particles.
Post-treatment evaluation
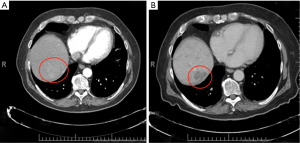
Tumor response was assessed by post-treatment CT scan (Figure 3). Response was determined according to guidelines from RECIST 1.1 (17,18). CR was defined as the disappearance of any intra-tumoral arterial enhancement in all target lesions. PR was defined as an at least 30% decrease in the sum of the diameters of viable target lesions. PD was defined as an at least 20% increase in diameters of enhancing target lesions or evidence of new lesion. SD was defined as any tumor response between PR and PD. If a patient expired before repeat imaging could be performed, they were considered to have PD.
Clinical and laboratory toxicities
Patients were followed up with regular visits at regular intervals as determined by the treating provider. Clinical and laboratory adverse events were recorded from medical records, following the National Cancer Institute Common Terminology Criteria (NCI CTC) version 3.0. Toxicities were measured and followed for both clinical symptoms and derangement of laboratory measurements of total bilirubin, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and Alk Phos.
Statistical analyses
Overall survival was the primary endpoint used in this study defined as the time between the date of first treatment and date of death. Secondary endpoints included survival since diagnosis, as well as proportion of patients with CR, PR, SD, and PD. Data were analyzed using descriptive methods. Median overall survival (including corresponding 95% confidence interval) was determined through Kaplan-Meier survival-analysis. Statistical analysis was performed with the software program R (19).
Results
Baseline characteristics
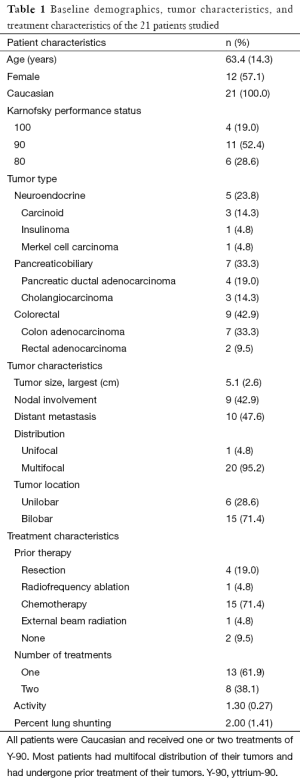
The 21 patients studied were predominantly between the ages of 50 and 75 years old and Caucasian (Table 1). The types of cancers studied were neuroendocrine (n=5), pancreaticobiliary tract (n=7), and colorectal (n=9). A majority of patients had undergone prior therapy, including chemotherapy (n=15), resection (n=4), and radiofrequency ablation (n=1). Most of the tumors were multifocal and bilobar, and in approximately half of the cases, the typical tumor size was 5 cm or smaller, commonly with extrahepatic and lymph node involvement.
Full table
Clinical and laboratory toxicities
The most common clinical toxicities were abdominal pain in 9 (43%) patients, followed by nausea and vomiting in 8 patients (38%). Anorexia and fatigue occurred in only 3 (14%) and 4 (19%) patients, respectively, and diarrhea occurred in only 2 cases (10%). About 33% of patients experienced no clinical side effects whatsoever.
The most common toxicity measured by laboratory values was Alk Phos, which showed mild toxicity in 11 patients, or 52% of our cohort (Table 2). Mild AST and ALT toxicities occurred in 6 and 7 patients respectively (29% and 33%). Bilirubin toxicity was mild in 3 patients (14%) and severe in 1 case (5%), and albumin toxicity was mild in 3 cases (14%) and severe in only 1 case (5%).
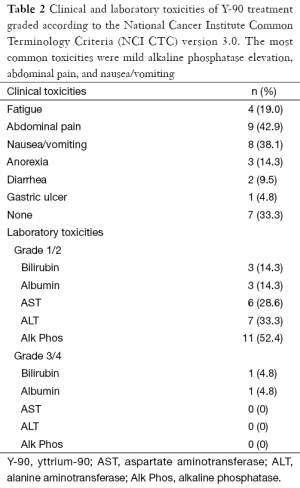
Full table
Tumor response
In this 21 patient cohort, 15 patients (71%) experienced a clinical benefit (either SD or PR). Eight patients (38%) experienced SD, and 7 patients (33%) experienced a PR (Table 3). A CR was not seen in any of these cases.
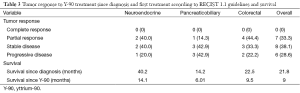
Full table
For patients with neuroendocrine tumors (n=6), 2 patients had a PR, 2 patients had SD, and 1 patient had progression of disease; the clinical benefit was 80%. Of the pancreatobiliary tumors (n=7), 3 patients had SD, and a single patient had a PR, for a total of 57% clinical benefit. Colorectal cancer (n=9) had a higher clinical benefit of 78% (3 with SD; 4 with PR).
Survival
Overall survival for this patient cohort was 22.5 months. Survival since the time of diagnosis for patients with neuroendocrine, pancreaticobiliary, and colorectal tumors were 40.2, 14.2, and 22.5 months respectively (Table 3). Kaplan-Meier curves for overall survival from diagnosis are demonstrated in Figure 4.
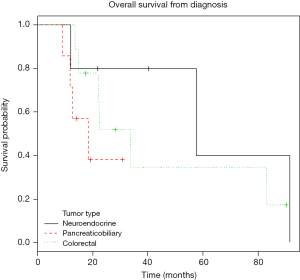
Prognosis
Unsurprisingly, the presence of increased index tumor size, extrahepatic metastases, and derangements in any lab value prior to treatment were significant predictors of poorer prognosis and survival rate (Table 4). However, the presence or absence of neoplasm-invaded lymph nodes did not seem to strongly correlate with survival.
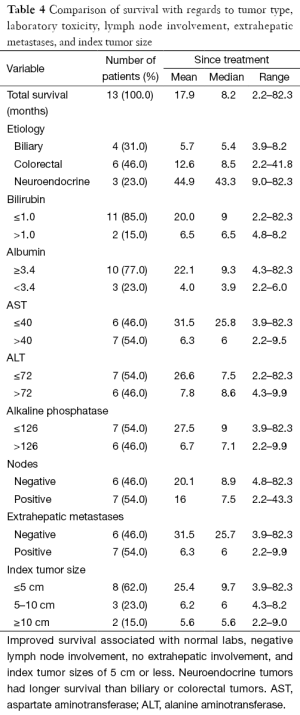
Full table
While derangement of any of five lab values (total bilirubin, serum albumin, AST, ALT, and Alk Phos) would be a strong predictor of poorer outcome, total bilirubin and serum albumin were abnormal in very few of our patients prior to treatment.
Conclusions
The growing literature on radioembolization shows that Y-90 is an effective treatment for the management of metastatic tumors (20). The median survival of chemorefractory, metastatic liver disease from colorectal, neuroendocrine, and biliary cancer after treatment with Y-90 radioembolization in our retrospective cohort was 9.5, 14.1, and 6.01 months, respectively. This is comparable to median survival times of 4.5–17 months for metastatic colorectal cancer and 23–45 months for metastatic neuroendocrine tumors (21-23). Overall median survival after treatment with Y-90 radioembolization in the biliary tract group varied depending on the specific primary malignancy; however a median survival 11.3–16.3 months for patients with intrahepatic cholangiocarcinoma has been reported in other similar studies (24,25).
A clinical benefit, defined as the presence of CR, PR, or SD by RECIST criteria, was seen in 71% of patients in our cohort. A 78% clinical benefit was seen in the metastatic colorectal cancer subgroup, consistent with prior studies (13). A 57% clinical benefit was seen in the metastatic biliary tract cancer subgroup, and an 80% clinical benefit was seen in the metastatic neuroendocrine cancer subgroup; however, a clinical benefit has been previously reported as high as 90% in metastatic disease to the liver from primary sites other than colorectal cancer (26). CR in metastatic liver disease is exceedingly uncommon as demonstrated in our series, consistent with prior reports. Evaluation of treatment response by mRECIST criteria involves monitoring changes in lesion size post treatment. These radiographic changes may not reflect the true effectiveness of treatment due to changes that occur after Y-90 radioembolization such as hemorrhage, peritumoral edema, and ring enhancement within and surrounding the tumor site (27). Cianni et al. suggest that tumor response may be better determined with a volumetric assessment, using FDG-PET or fMRI with diffusion-weighted sequences as a means to evaluate tumor response.
This sample of patients confirms that Y-90 radioembolization is a generally safe and well-tolerated procedure. The majority of the laboratory toxicities noted in our study included low-grade derangements in albumin, transaminase, bilirubin and Alk Phos levels. The most common clinical toxicities were abdominal pain in 9 patients (43%), followed by nausea and vomiting in 8 patients (38%). These symptoms are consistent with the well-described complication of radioembolization known as post embolization syndrome (PES). Symptoms of PES include nausea, fevers, right upper quadrant pain, and vomiting; given these findings, PES is most likely the cause of the clinical toxicities experienced by our cohort (28). Radioembolization can cause serious complications including gastrointestinal ulceration, pancreatitis and cholecystitis (29); however, these complications were not experienced in our patient sample.
Radioembolization has been shown to be comparable to other locoregional therapies as a salvage regimen for metastatic liver disease. Hong et al. reported a similar survival benefit and fewer complications with radioembolization compared to chemoembolization for liver-dominant metastatic colorectal adenocarcinoma in 36 patients (30). To date, no randomized controlled trials have compared radioembolization and chemoembolization for cholangiocarcinoma. However, trials are currently underway comparing the two treatment approaches as a first line therapy for inoperable cholangiocarcinoma confined to the liver (31). A twenty-article meta-analysis evaluating median overall survival and tumor response to therapy in patients with unresectable intrahepatic cholangiocarcinoma showed that median overall survival was 22.8 months with hepatic arterial infusion (HAI), 13.9 months in Y-90 radioembolization, and 12.4 months in transcatheter arterial chemoembolization (TACE). Additionally, Y-90 radioembolization response rates (27.4%) were inferior to HAI (56.9%), but superior to TACE (17.3%) (32). For metastatic neuroendocrine tumors within the liver, several studies suggest that radioembolization may be the preferred option over other intra-arterial based therapies. Studies of moderate quality evidence evaluated by a multidisciplinary group of experts suggest that radioembolization may cause fewer side effects compared to transcatheter arterial embolization (TAE) and TACE; however the quality and strength of the reports from this study were limited when comparing survival, symptomatic response, and imaging response (33).
There were several limitations to our study. Our small sample size, lack of control or comparison group, and heterogeneous primary cancer types within the study limits the ability to generalize our findings. Additionally, a majority of patients in our cohort had multifocal metastatic disease, which is a poor prognostic indicator; however this factor is inherent to the patient population typically selected for radioembolization. Despite these limitations, our data adds to the growing body of evidence suggesting that radioembolization is a reasonable treatment option to stabilize or improve the burden of metastatic liver disease in patients with chemorefractory liver metastases and is generally well tolerated.
Acknowledgments
We would like to thank our staff and colleagues at Scripps Green Hospital and Scripps Clinic.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2016.01.13). HG serves as an unpaid Co-Editor-in-Chief of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Data were collected retrospectively. The study was approved by our institutional review board, and patient data were deidentified compliant with the Health Insurance Portability and Accountability Act. Individual consent was waived. This work conforms to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ackerman NB, Lien WM, Kondi ES, et al. The blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to "small" and "large" tumors. Surgery 1969;66:1067-72. [PubMed]
- Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg 1989;76:545-8. [PubMed]
- Kennedy AS, Nutting C, Coldwell D, et al. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60:1552-63. [PubMed]
- Fox RA, Klemp PF, Egan G, et al. Dose distribution following selective internal radiation therapy. Int J Radiat Oncol Biol Phys 1991;21:463-7. [PubMed]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006;12:895-904. [PubMed]
- Gilbert HA, Kagan AR. Metastases: Incidence, detection, and evaluation without histologic confirmation. In: Weiss L, editor. Fundamental Aspects of Metastasis. Amsterdam: North-Holland Pub. Co., 1976:385-405.
- Brodt P. Introduction. In: Brodt P, editor. Liver metastasis: Biology and Clinical Management. Berlin: Springer Science and Business Media, 2011:1-5.
- Fox AM, Gallinger S, Moulton CA. Colorectal Carcinoma Liver Metastasis: Surgical Clinical Perspective. In: Brodt P, editor. Liver metastasis: Biology and Clinical Management. Berlin: Springer Science and Business Media, 2011:353-80.
- Poston GJ, Adam R, Alberts S, et al. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol 2005;23:7125-34. [PubMed]
- Welsh JS, Kennedy AS, Thomadsen B. Selective Internal Radiation Therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma. Int J Radiat Oncol Biol Phys 2006;66:S62-73. [PubMed]
- Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet 1994;343:1405-10. [PubMed]
- Knox CD, Pinson CW. Liver Metastases from Endocrine Tumors. In: Clavien PA, editor. Malignant Liver Tumors: Current and Emerging Therapies. West Sussex: John Wiley & Sons, 2010:424-38.
- Saxena A, Bester L, Shan L, et al. A systematic review on the safety and efficacy of yttrium-90 radioembolization for unresectable, chemorefractory colorectal cancer liver metastases. J Cancer Res Clin Oncol 2014;140:537-47. [PubMed]
- Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. [PubMed]
- Cao CQ, Yan TD, Bester L, et al. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg 2010;97:537-43. [PubMed]
- Townsend A, Price T, Karapetis C. Selective internal radiation therapy for liver metastases from colorectal cancer. Cochrane Database Syst Rev 2009;CD007045 [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
The R Project for Statistical Computing - Kennedy A, Coldwell D, Sangro B, et al. Radioembolization for the treatment of liver tumors general principles. Am J Clin Oncol 2012;35:91-9. [PubMed]
- Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer 2005;104:1590-602. [PubMed]
- John PK, Saif MW. Radioembolization in the treatment of neuroendocrine tumors of the pancreas. JOP 2014;15:332-4. [PubMed]
- Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 2009;19:951-9. [PubMed]
- Camacho JC, Kokabi N, Xing M, et al. Modified response evaluation criteria in solid tumors and European Association for The Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol 2014;25:256-65. [PubMed]
- Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol 2013;20:3779-86. [PubMed]
- Bangash AK, Atassi B, Kaklamani V, et al. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol 2007;18:621-8. [PubMed]
- Cianni R, Urigo C, Notarianni E, et al. Radioembolisation using yttrium 90 (Y-90) in patients affected by unresectable hepatic metastases. Radiol Med 2010;115:619-33. [PubMed]
- Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006;29:522-9. [PubMed]
- Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 2009;20:1121-30; quiz 1131. [PubMed]
- Hong K, McBride JD, Georgiades CS, et al. Salvage therapy for liver-dominant colorectal metastatic adenocarcinoma: comparison between transcatheter arterial chemoembolization versus yttrium-90 radioembolization. J Vasc Interv Radiol 2009;20:360-7. [PubMed]
- Kloeckner R, Ruckes C, Kronfeld K, et al. Selective internal radiotherapy (SIRT) versus transarterial chemoembolization (TACE) for the treatment of intrahepatic cholangiocellular carcinoma (CCC): study protocol for a randomized controlled trial. Trials 2014;15:311. [PubMed]
- Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol 2015;111:213-20. [PubMed]
- Kennedy A, Bester L, Salem R, et al. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford) 2015;17:29-37. [PubMed]


