
Emerging roles of FGF signaling in hepatocellular carcinoma
Introduction
Liver cancer is the fifth most common malignance in the world (1). It has been known that 90% of liver cancers are hepatocellular carcinoma (HCC). It is estimated that more than 740,000 new cases of HCC were diagnosed and approximately 700,000 deaths were occurred worldwide (1). In the USA, 35,660 people will be diagnosed with HCC and 24,550 patients will die from HCC in year 2015 (2). In China, more than 400,000 new cases will be developed and 370,000 patients will die due to this deadly disease (3). Although surgical resection and liver transplantation have been improved, the 5-year survival rate has no significant decrease in part due to the fact that most patients are in the late stage at diagnosis (4). Additionally, many HCC patients exhibit low sensitivity to standard radiotherapy and chemotherapy (5,6). For example, sorafenib is the standard drug for the treatment of advanced HCC cases; however, the median overall survival of these HCC patients is still less than 1 year partly due to drug resistance (7). Therefore, it is pivotal to discover the new therapeutic drugs for the treatment of HCC patients (8,9). Emerging evidence has suggested that hepatitis C virus (HCV) and hepatitis B virus (HBV) infection are important risk factors for the incidence of HCC (10,11). In addition, obesity, diabetes and nonalcoholic steatohepatitis have been found to contribute to HCC incidence (12). Without a doubt, the presence of cirrhosis is the overriding risk factor for HCC. In recent years, several lines of evidence has defined that some genes and cellular signaling pathways play a key role in the development and progression of HCC, including Notch, PI3K (phosphatidylinositol 3-Kinase)/Akt, extracellular-regulated kinase (ERK), mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK), Hedeghog, and Wnt (13-18) pathways. Moreover, some growth factor signaling pathways such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), transforming growth factor (TGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) have also been emerged as critical players in tumorigenesis including liver carcinogenesis (19-21). Among some of these pathways, FGF has recently gained high attention in HCC development and progression (22). Therefore, in this article, we will briefly describe recent advances in the physiological function and molecular mechanism of FGF in HCC. We also present the current chemical inhibitors of FGF/FGFR and natural agents that inactivate FGF signaling pathway. Lastly, we will discuss whether FGF/FGFR could be the prognostic markers and/or potential targets for the treatment of HCC patients.
FGF signaling pathway
FGF was identified forty years ago and has been extensionally studied over the last three decades (23). There are 22 human FGFs, which are encoded by different genes. It has been known that most FGFs are secreted and contain signal-peptide sequences (23). Structurally, the FGF protein has FGFR-binding domains and HS (heparin sulfate)-binding domains, which is required for FGFR dimerization and activation (23). There are four types of FGFRs (FGFR1, 2, 3, 4) that have been identified to function as RTKs (receptor tyrosine kinases) (24). Specifically, FGFRs family contains FGFR1b, FGFR1c, FGFR2b, FGFR2c, FGFR3b, FGFR3c, and FGFR4 (25). FGFR proteins consist of extracellular immunoglobulin-like domains and the cytoplasmic tyrosine kinase domain. It has been demonstrated that FGFs function as ligands that bind to multiple FGFRs, leading to the autophosphorylation of FGFR at an important tyrosine residue and subsequent activation of its tyrosine kinase domain (26). The activated receptor signals exert their physiological functions through multiple downstream pathways such as RAS-MAPK, PI3K-AKT or PLCγ. Interestingly, FGF-mediated RAS-MAPK activation mainly regulates cellular proliferation, while FGF-trigged PI3K-AKT activation largely controls cellular survival (26). FGF signaling pathway was initially studied in wound healing in skin. In recent years, the FGF pathway was found to also play a critical role in carcinogenesis including HCC (27-33). In the following paragraphs, we will describe an overview of this growth factor pathway in the development and progression of HCC, which have largely burst onto the scene.
The role of FGF in HCC
Recent some studies have highlighted the important role of FGF in HCC progression and metastasis. For example, FGF2 expression was only detected in the liver tissues of patients with chronic hepatitis (CH) type C and HCC, but not in normal liver tissue (34). Similarly, the serum FGF2 levels were higher in patients with CH, liver cirrhosis (LC) or HCC compared with healthy volunteers (34). Interestingly, the serum FGF2 levels were largely associated with the progression of chronic liver disease. Further study indicates that HCC cells could produce FGF2 to eliminate HCC cells by innate immunity (34). Another independent study showed that at least one member of the FGF8 subfamily (FGF8, FGF17, and FGF18) was up-regulated in HCC patients (35). Consistently, the levels of their corresponding receptors (FGFR2, FGFR3, and FGFR4) were also increased in HCC samples. Mechanistically, down-regulation of FGF18 by its siRNA inhibited cell proliferation, whereas FGF8, FGF17, and FGF18 induced the cell proliferation and tube formation (35). Thus, FGF8, FGF17, and FGF18 enhanced the survival of HCC cells, suggesting that they are critically involved in the development and progression of HCC (35). Furthermore, FGF15 has been known as a gut-derived hormone that regulates bile acids and carbohydrate metabolism. FGF15 is involved in liver regeneration after partial hepatectomy through enhancing hepatocellular proliferation. Depletion of Fgf15 in mice showed less and smaller tumors after these mice underwent carcinogenesis (36). Furthermore, this study further demonstrated that FGF15 might contribute to HCC development in a context of chronic liver injury and fibrosis (36).
Notably, ectopic expression of FGF19 in mice corrects bile acid signaling defects in a transgenic mouse model, leading to the size of the bile acid pool and liver size in mice comparable with humanized livers (37). On the other hand, Wu et al. reported that FGF19 can increase hepatocyte proliferation and induce HCC formation (38). Further observation identified that amino acids residues at 38–42 of FGF19 are sufficient to confer both FGFR4 activation and increased hepatocyte proliferation (38). Moreover, FGF19 promotes epithelial-mesenchymal transition (EMT) in HCC cells through modulating the GSK3β/β-catenin signaling cascade via FGFR4 activation (39). Specifically, overexpression of FGF19 promoted EMT and invasion in HCC cells via repression of E-cadherin expression, whereas depletion of FGF19 enhanced E-cadherin expression and diminished EMT traits. Moreover, the expression of FGF19 is significantly elevated and negatively associated with E-cadherin expression in HCC tissues and cell lines (39). It has also been found that FGF19 recombinant protein could increase the proliferation and invasion capabilities of HCC cells and inhibit their apoptosis (40). However, down-regulation of FGF19 expression by its siRNA significantly suppressed proliferation and induced apoptosis in HCC cells (40). Furthermore, one study demonstrated that the progeny of FGF19 transgenic mice developed HCC (41). Importantly, it has been reported that FGFR4 is required for hepatocarcinogenesis because FGF19 transgenic mice failed to develop liver tumors after they bred with FGFR4 knockout mice (41). Miura et al. examined the expression of FGF19 in HCC specimens and found that FGF19 was significantly overexpressed in HCCs as compared with corresponding noncancerous liver tissue (40). Moreover, FGF19 mRNA expression was an independent prognostic factor for overall and disease-free survival in HCC patients (40).
FGFR mediated survival and proliferation of murine hepatoblasts and hepatic tumor initiating stem cells in part through activation of AKT-β-catenin-CBP pathway (42). Another study identified that FGFR4 expression is elevated in several types of cancers including liver cancer, suggesting that FGFR4 could be an important and novel therapeutic target in HCC (41). Consistently, inhibition of FGFR4 expression by its siRNA induced apoptosis and retarded proliferation in HCC cells (40). However, Huang et al. revealed that FGFR1 is a strong promoter of hepatoma due to depressing cell death, whereas FGFR4 serves to suppress hepatoma progression (43). Specifically, FGFR4-deficient mice accelerated diethylnitrosamine (DEN)-initiated hepatocarcinogenesis. Mechanistically, FGFR4 failed to sustain ERK activation and did not trigger AKT pathway (43). In keeping with this finding, FGFR-1 was overexpressed in HCC, suggesting that FGFR-1 is potential prognostic markers and therapeutic targets in HCC (44). However, further investigation is necessary to explore the role of various FGFR isoforms in HCC progression.
FGF as a HCC cancer therapeutic target
Given the range of important functions of FGF in HCC progression, it is pivotal to find the novel strategy to modify the FGF/FGFR signaling pathway. Specific antibodies targeted against the members of FGF family could be useful. For instance, an anti-FGF19 monoclonal antibody that selectively blocks the interaction of FGF19 with FGFR4, effectively prevented HCC in FGF19 transgenic mice due to inhibition of FGF19-dependent activation of FGFR4, FRS2, ERK and beta-catenin (45). Another independent study also validated that anti-FGF19 antibody led to reduction of growth of colon tumor xenografts and prevents HCC in FGF19 transgenic mice (46). Similarly, LD1, a blocking anti-FGFR4 monoclonal antibody, inhibited FGF1 and FGF19 binding to FGFR4, leading to inhibition of colony formation, proliferation, and tumor growth in a mouse model of liver cancer (41). However, antibody-mediated inhibition of FGF19 treatment increased bile acids synthesis and malabsorption of bile acids in cynomolgus monkeys, leading to severe diarrhea and low food consumption (46).
There is growing interest in the development of chemical inhibitors of the FGF/FGFR pathway. For example, LY-2874455 could potently inhibit all FGFRs and BGJ398 is potent and selective inhibitor of FGFR1-3 kinases (47). SSR128129E (SSR), a small molecule allosteric inhibitor of FGFR, binds to the extracellular part of the receptor. Interestingly, SSR inhibits FGF-induced signaling linked to FGFR internalization, but not compete with FGF for binding to FGFR (48). BLU9931, a potent, paralog-selective, and irreversible inhibitor of FGFR4, inhibited FGFR4 signaling and suppressed proliferation in HCC cell lines with an activated FGFR4 signaling pathway (47). More importantly, BLU9931 showed remarkable anti-tumor activity in mice bearing an HCC tumor xenograft with FGF19 overexpression (47). Brivanib attenuated hepatic fibrosis in vivo and decreased stellate cell activation in vitro through inhibition of FGF, VEGF and PDGF signaling, indicating that brivanib is a selective inhibitor of VFGFR and FGFR, which could be useful for the treatment of liver fibrosis and prevention of HCC (49). Similarly, brivanib was found to induce growth inhibition, apoptosis and cell cycle arrest through targeting VEGFR2, FGFR1, ERK and AKT phosphorylation (50). On the other hand, Dovitinib treatment led to significant anti-tumor and anti-metastatic activities partly through inhibition of FGF-induced phosphorylation of FGFR-1 in HCC xenograft models (51). Additionally, natural agent EGCG (epigallocatechin-gallate) increased the animal survival and decreased cell viability in HCC partly through reducing expression of FGF-2 (52). In addition, Suramin exerts its anti-tumor activity as well as hepatoprotective effects in part via inhibition of FGF-2 expression (53).
Conclusions and overall perspectives
In conclusion, FGF plays a critical role in the development and progression of human HCC due to that FGF governs various cellular processes such as cell proliferation, migration, invasion, angiogenesis, drug resistance and metastasis (Figure 1). FGF/FGFR has been identified to exert its physiological function through governing its downstream targets. Thus, targeting FGF/FGFR could be a potential approach for the treatment of HCC. However, further in-depth investigation is required to address some questions. For example, how to target FGF/FGFR in the specific organism, with minimal effect on the entire organism? Which molecules in FGF/FGFR signaling pathways are more important as the therapeutic targets? How to target downstream of FGF/FGFR interactions? How to use combination therapies to maximize the success of future clinical treatments?
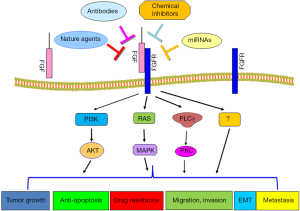
Recent studies demonstrated that miR-214 could inhibit liver cancer cell invasion through downregulation of FGFR1 expression (44). Consistently, downregulation of miR-214 and overexpression of FGFR1 were observed in HCC samples. This study indicates that upregulation of miR-214 could be an alternative approach to inhibit the expression of FGFR1 for the treatment of HCC (44). However, RNA-based drugs often have off-target effects and have no good delivery method yet. Since antibodies, peptides, and inhibitors could have toxic side-effects, natural compounds without non-toxic nature could be safer agents to inactivate FGF signaling pathway for treating HCC, which warrant further in-depth investigation.
Acknowledgments
Funding: This work was supported in part by the NIH grants to W.W. (GM094777 and CA177910). This work was also supported by grant from NSFC (81172087, 81572936) and the priority academic program development of Jiangsu higher education institutions.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2016.01.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians 2015;65:5-29. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Trovato FM, Tognarelli JM, Crossey MM, et al. Challenges of liver cancer: Future emerging tools in imaging and urinary biomarkers. World J Hepatol 2015;7:2664-75. [PubMed]
- Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015;12:408-24. [PubMed]
- Wörns MA, Galle PR. HCC therapies--lessons learned. Nat Rev Gastroenterol Hepatol 2014;11:447-52. [PubMed]
- Cabibbo G, Petta S, Maida M, et al. Sorafenib for Hepatocellular Carcinoma: From Randomized Controlled Trials to Clinical Practice. Dig Dis 2015;33:668-74. [PubMed]
- Schlachterman A, Craft WW Jr, Hilgenfeldt E, et al. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol 2015;21:8478-91. [PubMed]
- Singh S, Singh PP, Roberts LR, et al. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2014;11:45-54. [PubMed]
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013;10:553-62. [PubMed]
- Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer 2013;13:123-35. [PubMed]
- Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 2010;7:448-58. [PubMed]
- Chen C, Wang G. Mechanisms of hepatocellular carcinoma and challenges and opportunities for molecular targeted therapy. World J Hepatol 2015;7:1964-70. [PubMed]
- Meng X, Franklin DA, Dong J, et al. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res 2014;74:7161-7. [PubMed]
- Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology 2015;61:382-92. [PubMed]
- Matter MS, Decaens T, Andersen JB, et al. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol 2014;60:855-65. [PubMed]
- Moeini A, Cornella H, Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer 2012;1:83-93. [PubMed]
- Zheng X, Zeng W, Gai X, et al. Role of the Hedgehog pathway in hepatocellular carcinoma. Oncol Rep 2013;30:2020-6. [PubMed]
- Enguita-Germán M, Fortes P. Targeting the insulin-like growth factor pathway in hepatocellular carcinoma. World J Hepatol 2014;6:716-37. [PubMed]
- Yoshida K, Murata M, Yamaguchi T, et al. TGF-beta/Smad signaling during hepatic fibro-carcinogenesis. Int J Oncol 2014;45:1363-71. [PubMed]
- Höpfner M, Schuppan D, Scherübl H. Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J Gastroenterol 2008;14:1-14. [PubMed]
- Sandhu DS, Baichoo E, Roberts LR. Fibroblast growth factor signaling in liver carcinogenesis. Hepatology 2014;59:1166-73. [PubMed]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010;10:116-29. [PubMed]
- Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol 2013;14:166-80. [PubMed]
- Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev 2015;29:1463-86. [PubMed]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009;8:235-53. [PubMed]
- Hierro C, Rodon J, Tabernero J. Fibroblast Growth Factor (FGF) Receptor/FGF Inhibitors: Novel Targets and Strategies for Optimization of Response of Solid Tumors. Semin Oncol 2015;42:801-19. [PubMed]
- Criscitiello C, Esposito A, De Placido S, et al. Targeting fibroblast growth factor receptor pathway in breast cancer. Curr Opin Oncol 2015;27:452-6. [PubMed]
- Tiseo M, Gelsomino F, Alfieri R, et al. FGFR as potential target in the treatment of squamous non small cell lung cancer. Cancer Treat Rev 2015;41:527-39. [PubMed]
- Abdel-Rahman O. Targeting FGF receptors in colorectal cancer: from bench side to bed side. Future Oncol 2015;11:1373-9. [PubMed]
- Fearon AE, Gould CR, Grose RP. FGFR signalling in women's cancers. Int J Biochem Cell Biol 2013;45:2832-42. [PubMed]
- Corn PG, Wang F, McKeehan WL, et al. Targeting fibroblast growth factor pathways in prostate cancer. Clin Cancer Res 2013;19:5856-66. [PubMed]
- Kelleher FC, O'Sullivan H, Smyth E, et al. Fibroblast growth factor receptors, developmental corruption and malignant disease. Carcinogenesis 2013;34:2198-205. [PubMed]
- Tsunematsu H, Tatsumi T, Kohga K, et al. Fibroblast growth factor-2 enhances NK sensitivity of hepatocellular carcinoma cells. Int J Cancer 2012;130:356-64. [PubMed]
- Gauglhofer C, Sagmeister S, Schrottmaier W, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology 2011;53:854-64. [PubMed]
- Uriarte I, Latasa MU, Carotti S, et al. Ileal FGF15 contributes to fibrosis-associated hepatocellular carcinoma development. Int J Cancer 2015;136:2469-75. [PubMed]
- Naugler WE, Tarlow BD, Fedorov LM, et al. Fibroblast Growth Factor Signaling Controls Liver Size in Mice With Humanized Livers. Gastroenterology 2015;149:728-40. [PubMed]
- Wu X, Ge H, Lemon B, et al. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J Biol Chem 2010;285:5165-70. [PubMed]
- Zhao H, Lv F, Liang G, et al. FGF19 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells by modulating the GSK3β/β- catenin signaling cascade via FGFR4 activation. Oncotarget 2015; [Epub ahead of print].
- Miura S, Mitsuhashi N, Shimizu H, et al. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 2012;12:56. [PubMed]
- French DM, Lin BC, Wang M, et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One 2012;7:e36713 [PubMed]
- Mavila N, James D, Utley S, et al. Fibroblast growth factor receptor-mediated activation of AKT-beta-catenin-CBP pathway regulates survival and proliferation of murine hepatoblasts and hepatic tumor initiating stem cells. PLoS One 2012;7:e50401 [PubMed]
- Huang X, Yang C, Jin C, et al. Resident hepatocyte fibroblast growth factor receptor 4 limits hepatocarcinogenesis. Mol Carcinog 2009;48:553-62. [PubMed]
- Wang J, Li J, Wang X, et al. Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun 2013;439:47-53. [PubMed]
- Desnoyers LR, Pai R, Ferrando RE, et al. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene 2008;27:85-97. [PubMed]
- Pai R, French D, Ma N, et al. Antibody-mediated inhibition of fibroblast growth factor 19 results in increased bile acids synthesis and ileal malabsorption of bile acids in cynomolgus monkeys. Toxicol Sci 2012;126:446-56. [PubMed]
- Hagel M, Miduturu C, Sheets M, et al. First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway. Cancer Discov 2015;5:424-37. [PubMed]
- Herbert C, Schieborr U, Saxena K, et al. Molecular mechanism of SSR128129E, an extracellularly acting, small-molecule, allosteric inhibitor of FGF receptor signaling. Cancer Cell 2013;23:489-501. [PubMed]
- Nakamura I, Zakharia K, Banini BA, et al. Brivanib attenuates hepatic fibrosis in vivo and stellate cell activation in vitro by inhibition of FGF, VEGF and PDGF signaling. PLoS One 2014;9:e92273 [PubMed]
- Huynh H, Ngo VC, Fargnoli J, et al. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res 2008;14:6146-53. [PubMed]
- Huynh H, Chow PK, Tai WM, et al. Dovitinib demonstrates antitumor and antimetastatic activities in xenograft models of hepatocellular carcinoma. J Hepatol 2012;56:595-601. [PubMed]
- Darweish MM, Abbas A, Ebrahim MA, et al. Chemopreventive and hepatoprotective effects of Epigallocatechin-gallate against hepatocellular carcinoma: role of heparan sulfate proteoglycans pathway. J Pharm Pharmacol 2014;66:1032-45. [PubMed]
- Tayel A, Abd El Galil KH, Ebrahim MA, et al. Suramin inhibits hepatic tissue damage in hepatocellular carcinoma through deactivation of heparanase enzyme. Eur J Pharmacol 2014;728:151-60. [PubMed]


