
Lysophosphatidic acid as a regulator of lymphocyte trafficking in the lymph nodes
Lysophosphatidic acid (LPA) and its receptors
LPA is a bioactive lysophospholipid that regulates multiple biological processes, including cell growth, survival, and migration (1). Lysophospholipids consist of a glycerol backbone, a phosphate group, and a single acyl chain. They are more soluble in water than are diacyl phospholipids, which are the major components of the cell membrane. Notably, the hydrophilicity of lysophospholipids allows them to function as intercellular signaling molecules. The term LPA does not reflect a single molecular species, but represents multiple related species with fatty acids of different lengths and degrees of saturation (Figure 1). LPA is found in plasma at a concentration of approximately 100 nM, and circulating LPA is mainly generated by the enzymatic action of the secreted lysophospholipase D, autotaxin (ATX)/ectonucleotide pyrophosphatase/phosphodiesterase-2 (ENPP-2), on lysophosphatidylcholine (LPC), the most abundant lysophospholipid in plasma (concentration, 100-200 µM). LPA is also produced from cell membrane-derived phosphatidic acid by the activities of phospholipase A1 (PLA1) and PLA2 (2). Once produced in the bloodstream, LPA is rapidly degraded into biologically inactive monoacylglycerol by membrane-bound lipid phosphate phosphatases (3) and then cleared from the circulation.
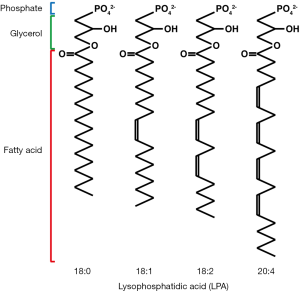
LPA binds specific receptors, including LPA1-6, which are all G-protein coupled receptors. These receptors are coupled to multiple G proteins, including Gi, G12/13, Gq, and Gs. Ligand binding to these receptors induces the activation of several intracellular signaling components, including the Ras, Rac and Rho GTPases, depending on which G proteins associate with the receptor. LPA1-3 was identified as members of the endothelial differentiation gene (EDG) family, which also includes S1P1, a receptor for another lysophospholipid, sphingosine-1-phosphate (S1P). LPA4-6 is newly discovered non-EDG family receptors, belonging to the purinergic P2Y receptor family (4). LPA also binds non-G-protein coupled receptors, including the receptor for advanced glycation end (RAGE) products (5), transient receptor potential cation channel V1 (TRPV1) (6), and the proliferator-activated receptor gamma (PPARγ) (7), although the biological significance of these interactions is unclear.
Generation of LPA by ATX/ENPP2
ATX/ENPP-2 is a member of the ENPP family. Other members of this family exhibit ATPase and ATP pyrophosphatase activities, and ENPP-3, ATX’s closest relative, converts mast cell- and basophil-derived ATP into ADP, which suppresses chronic allergic inflammation (8). In contrast, our data indicate that ATX is devoid of such activities (Yegutkin G and Miyasaka M, unpublished observation). ATX was originally identified as a cancer cell-derived autocrine motility factor, and was subsequently found to have lysophospholipase D activity, which converts LPC into LPA, and stimulates cell motility through LPA production (9). ATX-deficient mice die at E9.5-E10.5 due to defective vascular development in both the yolk sac and embryo proper (10-12). In contrast, we found that endothelial cell-specific ATX-deficient mice (Tie2-cre ATXfl/fl mice) survive without any obvious vascular abnormalities (Takeda A, unpublished data), indicating that endothelial cell-derived ATX is not essential for vascular development. ATX is also expressed in adipose tissues, and adipocyte-specific ATX-deficient mice (aP2-Cre ATXfl/fl mice) exhibit a moderate reduction in plasma LPA levels (to approximately 40% of wild-type levels), and after being fed a high-fat diet, they exhibit a higher fat mass and larger adipocyte size than control mice (13). The physiological role of adipocyte-derived LPA is currently unclear.
ATX is synthesized and N-terminally cleaved intracellularly in multiple cell types, and then exported through the classical secretory pathway. In some cases, ATX acts as an ectoenzyme on the cell surface by binding to glycosaminoglycan and integrins. ATX has four known splicing variants, ATXα, β, γ and δ. The predominant isoform is ATXβ, which is identical to plasma-borne lysophospholipase D (14). The longest isoform, ATXα, is the original cancer cell-derived motility-inducing factor, and is characterized by an insertion of 52 amino acid residues in its catalytic domain (15). These residues mediate its binding to negatively charged heparin, and ATXα, but not ATXβ, exhibits up to a two-fold higher lysophospholipase D activity after binding to heparin (15). Thus, depending on the ATX isoform produced, locally immobilized ATX may have stronger lysophospholipase D activity than soluble ATX.
Regulation of lymphocyte extravasation across endothelial cells
Emerging evidence suggests that the ATX/LPA axis is important in lymphocyte migration across high endothelial venules (HEVs) (16), which mediate the constitutive migration of lymphocytes from the circulating blood into the lymph nodes (17) (Figure 2). Both our group (18) and Kanda et al. (19) found that ATX mRNA is transcribed abundantly in the endothelial cells of HEVs. ATX expression appears 1 day after birth, which coincides with the postnatal recruitment of lymphocytes into the lymph nodes (18). This expression is independent of that of HEV-associated chemokines, including CCL21 and CXCL13, and is also unrelated to innate immunological signals mediated by TLR4 or MyD88 (18). In addition, ATX expression is at least partially regulated by lymphotoxin-β receptor signaling (Takeda A et al., submitted for publication), which also critically controls HEV differentiation and function (20). The major ATX isoform expressed in HEV endothelial cells is ATXβ (Takeda A, unpublished data), which does not bind heparin (15), as described above. Given that the constitutive association of ATX with the cell surface of HEV endothelial cells is cation independent (21), neither heparin/heparin sulfate nor integrins appear to anchor ATX to the HEV endothelial surface. Thus, the mechanism by which ATX associates with HEV endothelial cells is currently unknown.
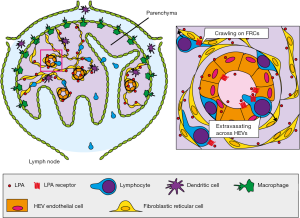
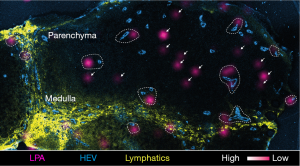
Using imaging mass spectrometry, which is a powerful tool for visualizing the distribution of lipids in tissues (22), we found that LPA is localized to the vicinity of HEVs in the lymph nodes (Figures 2,3). Among the LPA species, LPA 18:1, 18:2, and 20:4 which bind ATX strongly (23) are much more abundantly expressed in the lymph nodes than in skeletal muscle, whereas LPA 18:0, which binds ATX only weakly (23), is detected at comparable levels in these tissues (21), suggesting that the forms of LPA expressed in HEVs have an effect that is biologically relevant for that tissue.
Recent studies indicate that the ATX and LPA produced in HEVs regulate lymphocyte extravasation across these vessels. Kanda et al. showed that the intravenous injection of enzymatically inactive ATX inhibits HEV-mediated lymphocyte migration into the lymph node parenchyma (19), although it was unclear which step in lymphocyte extravasation (rolling, adhesion, or transmigration) is inhibited by the mutant ATX. Using intravital two-photon microscopy, we demonstrated that the local administration of ATX and/or LPA receptor inhibitors substantially inhibits lymphocyte transmigration across HEVs (21) and that local administration of LPA abrogates this effect, indicating that the ATX/LPA axis plays a role in regulating lymphocyte transmigration (21). In addition, using transmission electron microscopy, we found that ATX/LPA axis inhibitors induce lymphocyte accumulation in both the endothelial cell layer and the sub-endothelial compartment of HEVs, and that this accumulation is prevented by a local application of LPA (21). These findings suggest that the ATX/LPA axis regulates lymphocyte transmigration across the basal lamina of HEVs, but does not affect lymphocyte migration into the endothelial cell layer. In addition, the systemic inhibition of ATX by a specific inhibitor, HA-130, also attenuates the T cell migration across HEVs (24).
At least two mechanisms, which are not mutually exclusive, can be envisioned for how the ATX/LPA axis promotes lymphocyte extravasation across HEVs: one is that the ATX/LPA axis acts primarily on lymphocytes, and the other is that it acts on endothelial cells. Zhang et al. showed that treating naïve T cells with LPA or LPC plus ATX enhances their transendothelial migration across a mouse brain endothelial cell (bEnd.3) monolayer. In addition, they showed that ATX associates with the lymphocyte surface via Mn2+-activatable receptors, suggesting that ATX is anchored on the lymphocyte surface via integrin binding, and that ATX-produced LPA acts directly on the lymphocytes (24). However, the ATX binding to undisturbed naïve lymphocytes is minimal, and hence, the significance of ATX binding to naïve lymphocytes in lymphocyte trafficking across HEVs remains unclear. In contrast, our group reported that both LPC and LPA induce morphological changes in HEV endothelial cells; LPC’s effect is abrogated by ATX inhibition, whereas LPA’s effect is abrogated by ATX/LPA receptor inhibition, but not by ATX inhibition alone, in agreement with the previous finding that ATX is required to convert LPC to LPA. Using primary cultured HEV endothelial cells in an in vitro transmigration assay, we also found that ATX inhibition impairs the release of lymphocytes that migrate underneath HEV endothelial cells, and that this effect can be abrogated by adding LPA. The lymphocyte release from HEV endothelial cells is also attenuated by treating endothelial cells with the myosin II inhibitor blebbistatin (21). These findings indicate that LPA also acts on endothelial cells to induce lymphocyte release in a myosin II-dependent manner, thus regulating lymphocyte transmigration across the basal lamina of HEVs. HEV endothelial cells express LPA4 and LPA6, which are both G13 protein-coupled receptors. Recent results from our group indicate that LPA4-deficient endothelial cells specifically compromise the lymphocyte transmigration process, whereas the effect of LPA6-deficient endothelial cells is much milder, indicating that the signals evoked in HEV-endothelial cells via LPA4 and LPA6 differentially regulate lymphocyte extravasation across HEVs in the peripheral lymph nodes (Hata E et al., submitted for publication).
LPA’s role in interstitial lymphocyte migration within lymph nodes
Recently, Katakai et al. reported that ATX is also expressed in lymph node CCL21+ CD31− stromal cells. Using two-photon microscopic analysis of explanted lymph node tissue slices, they also showed that pharmacological inhibition of ATX/LPA reduces the velocity of T cell migration in the lymph node parenchyma (25), although they failed to show whether LPA directly promotes lymphocyte motility. On the other hand, we found that CCL19+ fibroblastic reticular cells (FRCs), along which lymphocytes migrate in a chemokine-dependent manner in the lymph node parenchyma (26), express ATX and produce LPA via ATX’s enzymatic activity. As shown in Figure 3, LPA is expressed in the lymph node paracortex at sites that are either close to or distant from HEVs, but it is only expressed marginally in the medulla, as revealed by imaging mass spectrometry of lymph node frozen sections. As mentioned earlier, certain LPA species, including LPA 18:1, 18:2, and 20:4 are produced preferentially in the lymph nodes (21). Notably, those biologically relevant species located at sites distal to HEVs are selectively reduced in FRC-specific ATX-deficient mice (CCL19-Cre ATXfl/fl mice) (27), in agreement with the hypothesis that FRCs produce LPA by the LPA-generating enzyme, ATX on their cell surface. Furthermore, intravital two-photon microscopic analysis showed that T cell migration in the parenchyma is significantly attenuated in the conditional ATX-deficient mice compared to that in control mice and that ATX/LPA-dependent T cell motility is mediated by the LPA receptor, LPA2, on the T cell surface (Takeda A et al.; submitted for publication). These results, together with the finding that LPA activates Rho-ROCK-myosin II pathways via LPA2 in lymphocytes, suggest that the LPA generated by FRCs acts locally on T cells via LPA2, thereby regulating T cell contractility and motility in the lymph node reticular network (Takeda A et al.; submitted for publication).
LPA’s roles in the migration of other immune cells and inflammation
LPA also plays important roles in the pathogenesis of inflammatory diseases, including arthritis, lung fibrosis, and asthma. Arthritic synovial fibroblasts express high levels of ATX, and treating the fibroblasts with LPA induces fibroblast proliferation, migration, and cytokine secretion in a manner that is at least partially dependent on LPA1-mediated signaling (28,29). The conditional ablation of ATX in mesenchymal cells (collagen IV-Cre ATXfl/fl) or LPA1-deficiency attenuates the development of arthritis in animal models (28,30). LPA levels in the bronchoalveolar lavage fluid increase following lung injury in the bleomycin-induced lung fibrosis model, and LPA1 deficiency attenuates fibroblast recruitment into the lung and the degree of fibrosis and mortality in this model (31). In addition, ATX and LPA levels are increased in the bronchoalveolar lavage fluid of patients or mice in response to airway allergen challenge, and ATX overexpression in mice accelerates allergic lung inflammation (32). The effect of LPA on lung inflammation appears to depend, at least in part, on LPA2, although LPA2 signaling has also been reported to have a negative effect on allergic lung inflammation (33).
Conclusions and perspectives
In this review, we discussed the roles of the ATX/LPA axis in lymphocyte transmigration across HEVs and in interstitial lymphocyte migration in the lymph node parenchyma, both of which are critical for proper adaptive immune responses. In addition, this axis also plays key roles in the pathogenesis of a number of inflammatory diseases. Given that lysophospholipid receptors involved in immune cell migration, such as the S1P receptors, have been successfully targeted for the treatment of inflammatory diseases, including multiple sclerosis (34), it is likely that ATX and the LPA receptors will also be therapeutically targeted for a variety of immunological disorders, including inflammation. Indeed, two LPA1 antagonists, SAR100842 and BMS986020, are currently in Phase 2 clinical trials in the U.S. for the treatment of systemic sclerosis and idiopathic pulmonary fibrosis, respectively (35). In addition, recent advances in our understanding of the physiological significance of other LPA receptors have prompted the development of many different types of LPA receptor antagonists, which are also being tested for clinical use. Thus, therapeutics targeting ATX and LPA receptors may play important roles in treating immunological/inflammatory disorders in the near future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hsinyu Lee and Markus H. Gräler) for the series “Lysophospholipids on Immunity and Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.09.03). The series “Lysophospholipids on Immunity and Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Choi JW, Herr DR, Noguchi K, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 2010;50:157-86. [PubMed]
- Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 2008;1781:513-8.
- Tomsig JL, Snyder AH, Berdyshev EV, et al. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem J 2009;419:611-8. [PubMed]
- Yanagida K, Kurikawa Y, Shimizu T, et al. Current progress in non-Edg family LPA receptor research. Biochim Biophys Acta 2013;1831:33-41.
- Rai V, Touré F, Chitayat S, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med 2012;209:2339-50. [PubMed]
- Nieto-Posadas A, Picazo-Juárez G, Llorente I, et al. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol 2011;8:78-85. [PubMed]
- Zhang C, Baker DL, Yasuda S, et al. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med 2004;199:763-74. [PubMed]
- Tsai SH, Kinoshita M, Kusu T, et al. The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity 2015;42:279-93. [PubMed]
- Nakanaga K, Hama K, Aoki J. Autotaxin--an LPA producing enzyme with diverse functions. J Biochem 2010;148:13-24. [PubMed]
- van Meeteren LA, Ruurs P, Stortelers C, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 2006;26:5015-22. [PubMed]
- Koike S, Keino-Masu K, Ohto T, et al. Autotaxin/lysophospholipase D-mediated lysophosphatidic acid signaling is required to form distinctive large lysosomes in the visceral endoderm cells of the mouse yolk sac. J Biol Chem 2009;284:33561-70. [PubMed]
- Tanaka M, Okudaira S, Kishi Y, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 2006;281:25822-30. [PubMed]
- Dusaulcy R, Rancoule C, Grès S, et al. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res 2011;52:1247-55. [PubMed]
- Tokumura A, Majima E, Kariya Y, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 2002;277:39436-42. [PubMed]
- Houben AJ, van Wijk XM, van Meeteren LA, et al. The polybasic insertion in autotaxin α confers specific binding to heparin and cell surface heparan sulfate proteoglycans. J Biol Chem 2013;288:510-9. [PubMed]
- Umemoto E, Hayasaka H, Bai Z, et al. Novel regulators of lymphocyte trafficking across high endothelial venules. Crit Rev Immunol 2011;31:147-69. [PubMed]
- Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 2004;4:360-70. [PubMed]
- Nakasaki T, Tanaka T, Okudaira S, et al. Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am J Pathol 2008;173:1566-76. [PubMed]
- Kanda H, Newton R, Klein R, et al. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol 2008;9:415-23. [PubMed]
- Browning JL, Allaire N, Ngam-Ek A, et al. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 2005;23:539-50. [PubMed]
- Bai Z, Cai L, Umemoto E, et al. Constitutive lymphocyte transmigration across the basal lamina of high endothelial venules is regulated by the autotaxin/lysophosphatidic acid axis. J Immunol 2013;190:2036-48. [PubMed]
- Goto-Inoue N, Hayasaka T, Zaima N, et al. Imaging mass spectrometry for lipidomics. Biochim Biophys Acta 2011;1811:961-9.
- Nishimasu H, Okudaira S, Hama K, et al. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat Struct Mol Biol 2011;18:205-12. [PubMed]
- Zhang Y, Chen YC, Krummel MF, et al. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J Immunol 2012;189:3914-24. [PubMed]
- Katakai T, Kondo N, Ueda Y, et al. Autotaxin produced by stromal cells promotes LFA-1-independent and Rho-dependent interstitial T cell motility in the lymph node paracortex. J Immunol 2014;193:617-26. [PubMed]
- Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 2012;12:762-73. [PubMed]
- Chai Q, Onder L, Scandella E, et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 2013;38:1013-24. [PubMed]
- Nikitopoulou I, Oikonomou N, Karouzakis E, et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J Exp Med 2012;209:925-33. [PubMed]
- Miyabe Y, Miyabe C, Iwai Y, et al. Activation of fibroblast-like synoviocytes derived from rheumatoid arthritis via lysophosphatidic acid-lysophosphatidic acid receptor 1 cascade. Arthritis Res Ther 2014;16:461. [PubMed]
- Miyabe Y, Miyabe C, Iwai Y, et al. Necessity of lysophosphatidic acid receptor 1 for development of arthritis. Arthritis Rheum 2013;65:2037-47. [PubMed]
- Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 2008;14:45-54. [PubMed]
- Park GY, Lee YG, Berdyshev E, et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med 2013;188:928-40. [PubMed]
- Emo J, Meednu N, Chapman TJ, et al. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J Immunol 2012;188:3784-90. [PubMed]
- Griffith JW, Luster AD. Targeting cells in motion: migrating toward improved therapies. Eur J Immunol 2013;43:1430-5. [PubMed]
- Kihara Y, Mizuno H, Chun J. Lysophospholipid receptors in drug discovery. Exp Cell Res 2015;333:171-7. [PubMed]


