
Sphingosine-1-phosphate (S1P) in cancer immunity and development
Introduction
Sphingosine-1-phosphate (S1P) is a lipid metabolite and signaling molecule that influences many different cellular functions. It is produced by two different sphingosine kinases, SphK1 and SphK2 (Figure 1) (1-3). Interestingly cells devoid of both SphKs are still able to grow and to proliferate, which demonstrates that S1P is not required for normal cell survival (4). Numerous studies however show that S1P can rescue cells from apoptosis and cell death induced by other stimuli, suggesting its potential role as a survival factor under certain pathologic conditions including cancer (5-15). Mice deficient of both SphK1 and SphK2 die around embryonic day 11.5 mainly due to vascular and neurologic defects, which emphasizes its role in developmental processes (4). While various different phosphatases like lipid phosphate phosphatases (LPPs) (16), sphingosine phosphate phosphatases (SPPs) (17,18) and also alkaline phosphatases are able to dephosphorylate S1P (19), which obviously is a rather unspecific event that can be initiated by many different enzymes, only the retro-aldolase S1P-lyase is able to cleave and irreversibly degrade S1P into hexadecenal and phosphoethanolamine (Figure 1) (20-23). The activity of anabolic SphKs on one side and catabolic phosphatases and the S1P-lyase on the other side together with the availability of the substrate sphingosine basically determine the cellular amount of S1P. The balance of intracellular S1P and ceramides determines cell fate (Figure 1) (24). More cellular S1P induces cell survival, while an increase in ceramides shifts cells into apoptosis. A potential reason for the survival effect of S1P could be the induction of autophagy (25). Accumulation of S1P in thymocytes from S1P-lyase deficient mice however cannot prevent apoptosis induced by increased ceramide levels (22). Therfore the interconversion of S1P and ceramides may be more relevant for cell fate decision than the total amounts of these metabolites present in cells, and this may well be adapted by cancer cells to increase survival. Current data indicate that SphK2 predominantly feeds into the catabolic metabolism determined by the activity of the S1P-lyase (26), while SphK1 is inducibly transported to the cell membrane to produce S1P that is subsequently secreted by cells (27). Spinster homolog 2 (Spns2) is a transporter integrated into the cell membrane that is involved in export of S1P from the cytosol into the extracellular matrix (28,29). S1P is an extracellular signaling molecule and ligand of five G protein-coupled S1P receptors designated S1P1-5 (30-33). S1P secretion can therefore lead to autocrine and paracrine signaling in the local tissue environment and also to endocrine signaling at distant locations via release into circulation. In fact S1P concentrations are highest in plasma and lymph, and very low in tissues due to the activity of the S1P-lyase which is predominantly expressed by tissue cells (21,22,34,35). These differences produce concentration gradients between the circulatory system and peripheral tissues which are important to induce lymphocyte egress from lymphoid organs and to maintain lymphocyte circulation (36-40). Neutralizing these S1P gradients by increasing the S1P concentration in lymphoid tissues prevents lymphocyte egress from thymus and lymph nodes and results in lymphopenia, although S1P as the primary exit signal is still present in blood at high micromolar levels (21,22,35,41). The reason for the unresponsiveness of lymphocytes to the exit signal S1P in blood and lymph is the premature downregulation of the S1P1 receptor (36,38). S1P is also a very potent inducer of angiogenesis and vascular barrier stability (42-45). The latter requires some kind of constitutive signaling activity that is also important for cancer cells, although under different circumstances. Constitutive signaling particularly of the nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) is frequently observed in cancer cells and under normal conditions prevented by receptor desensitization and internalization (46). How constitutive signaling of high blood S1P concentrations is possible is currently not clear (42). Recent data suggest a role of the S1P-lyase, at least for the endothelial barrier-stabilizing activity of S1P (47). Thus, many cancer-related functions of S1P are known like cell survival, autophagy, constitutive signaling, migration, cell differentiation, and development.
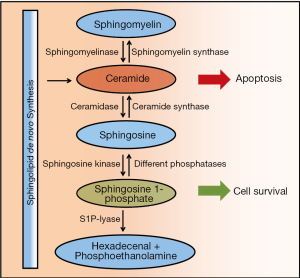
The sphingolipid rheostat
A key feature of tumor cells is unrestricted cell growth and enhanced survival, although isolated tumor cells are frequently more sensitive and die earlier than related normal primary cells when taken into cell culture, pointing to exogenous factors that promote tumor growth and survival in vivo (48). While early reports focused on sphingosine and its blocking activity on protein kinase C (PKC) (49,50), which can be attributed to its competition with the PKC activator phosphatidylserine (PS) for PKC binding, further studies revealed significant non-PKC mediated side effects that pointed to other relevant signaling events (51,52). Subsequently S1P and ceramides as the closest sphingosine metabolites came into the focus as potential mediators of sphingosine-related effects (53,54), although sphingosine itself still has unique physiological functions apart from ceramide and S1P signaling (55). Ceramide evolved as a pro-apoptotic molecule (56,57), while S1P was regarded as a pro-survival factor (8,9). These opposing physiological functions of the two closest metabolites of sphingosine shaped the concept of the sphingolipid rheostat (24). It postulates that the intracellular balance of ceramide and S1P generation determines cell fate (Figure 2). Increased ceramide production would lead to apoptosis while increased S1P production would promote cell survival (24). S1P-induced autophagy was later on identified as a possible mechanism to promote cell survival and to avoid ceramide-induced apoptosis (25,58). Cellular production of S1P by SphKs appears to be a key event of this hypothesis, and indeed many publications report increased expression particularly of SphK1 in different tumors (59). On the other hand, accumulation of S1P in thymocytes of S1P-lyase deficient mice did not prevent ceramide-induced apoptosis and thymus atrophy, indicating that the sole presence of high amounts of intracellular S1P was not sufficient to rescue them from cell death (22). In addition specific SphK inhibitors failed to inhibit tumor cell growth and viability, also questioning the relevance of the sphingolipid rheostat concept in cancer (60). Since intracellular targets of both S1P and ceramide are still not worked out very well, defining relevant intracellular signaling processes for both metabolites will be required to understand this system more thoroughly and to potentially use it for medical interventions.
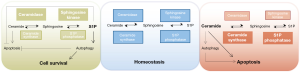
S1P receptor signaling
In contrast to ceramide, S1P is also an extracelluar signaling molecule and a ligand for five G protein-coupled cell surface receptors. Type 1 and type 2 S1P-receptors are particularly often described in the literature in the context of cancer. S1P1 signaling is frequently linked to promotion of tumor cell survival, providing an alternative pathway for S1P-mediated vitality (13,61). A major downstream signaling molecule of S1P1 is the serine/threonine kinase Akt (62-65). S1P1 is also known to induce cell migration, and several studies link S1P1 expression and function with tumor cell migration, invasion, and metastasis (66-68). Notably S1P2 appears to induce opposing effects at least on germinal center B cells by dampening Akt activation, growth, and S1P1-mediated migration (69,70). Loss of function mutations in S1P2 are thus linked with the occurrence of germinal center-like (GCB) diffuse large B cell lymphomas (DLBCL) (71,72). In contrast to the concept of the sphingolipid rheostat where SphKs play a major role for the intracellular generation of S1P, signaling of S1P receptors requires the presence of extracellular S1P, which can be produced locally by tumor cells via SphKs, but it can also be provided via the circulatory system from distant sources. Tumor angiogenesis is an important step in the development of solid cancers, and S1P is a strong angiogenic factor that mainly acts via activation of S1P1 on endothelial cells (45,73-77). Evidence exists that S1P1-mediated angiogenesis can also be opposed by S1P2 signaling (78).
Inside-out signaling
The concomitant presence of S1P receptors and SphKs in one cell shaped the concept of inside-out signaling (79,80). SphKs are intracellular enzymes, and in order to stimulate S1P receptors on the cell surface, S1P needs to be released by cells (Figure 3). This release is supported by ATP binding cassette (ABC) transporters, a large family of ubiquitously expressed integral membrane proteins that actively transport ligands across biological membranes (81). Although several reports indicate the involvement of ABC transporters in S1P exportation (82-86), other studies did not find a specific role of ABC transporters for S1P exportation from erythrocytes and endothelial cells (87,88). A clear function as a S1P-transporter however could be assigned to the membrane protein Spns2 (28,29). Recent reports indicate the involvement of Spns2 in tumor angiogenesis, cancer cell survival and migration (89,90). As mentioned before, many tumors upregulate expression of SphKs, particularly SphK1 (59), and it is an appealing hypothesis to suggest that tumor cells produce their own survival factor S1P that is secreted by ABC transporters and/or Spns2 into the tumor matrix to induce autocrine and paracrine survival signals via S1P-receptors, particularly S1P1 (Figure 3). But so far there are no reports available demonstrating increased S1P concentrations in tumors compared to normal tissues, and also the contribution of systemic S1P supply via the circulatory system is not clear. Blocking strategies for systemic S1P by either using an anti-S1P antibody (91) or an S1P-neutralizing L-aptamer (92) hold promise that systemic eradication of S1P could dampen tumor progression (45,93-99).
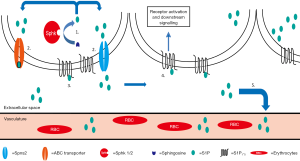
Adaptation
Adaptation is a process that allows cells to survive and proliferate under difficult conditions. A classical case of adaptation is the occurrence of drug resistance, and there is strong evidence that S1P and particularly the expression of SphK1 confers resistance of cancer cells to different therapeutic drugs and treatments (77,82,95,100-111). A common mechanistic pattern is S1P-mediated increase in vitality and prevention of ceramide-induced apoptosis (101,109) together with S1P receptor-mediated survival signaling (108,111). Another variant of adaptation is the establishment of constitutive NF-κB signaling via the S1P1-STAT3 axis (46,112). The signal transducer and activator of transcription-3 (STAT3) is a transcription factor for S1P1, so that S1P1 expression is elevated in STAT3-positive tumors. As a positive feedback-loop, upregulation of S1P1 activates STAT3 and results in increased interleukin-6 (IL6) production, which accelerates tumor growth and metastasis in a STAT3-dependent manner (46,112).
Immune escape mechanisms
Degenerated cells are usually detected and killed by immune cells. Obviously this endogenous defense system is not working properly anymore when tumors are formed. One reason for a failed immune response against tumors are certain immune escape strategies of the tumors that prevent either their recognition or their efficient attack by the immune system. Accumulation of regulatory T cells (Tregs) for example blocks the onset of an immune response, and S1P1 expression in T cells impairs their generation and their immune suppressive function (65,113). Under physiological conditions, increased expression of S1P1 therefore reduces the amount and activity of Tregs. Tumors however reverse the activity of S1P1 signaling in T cells by promoting the migration of Tregs into the tumor in a STAT3 dependent manner (114). Concomitantly activity and accumulation of CD8 T cells in tumors are reduced (114). There is also evidence that S1P released by apoptotic cells within tumors induce the generation of regulatory macrophages (14). These tumor-associated macrophages infiltrate tumors and promote tumor growth by, for example, activating hypoxia inducible factor 1α and releasing vascular endothelial growth factor (14).
Concluding remarks
Main functions of S1P include inhibition of apoptosis and induction of cell survival, cell migration, and angiogenesis. Tumor cells make use of this system to enhance their growth and proliferation, to invade tissues, to adapt to different environments, and to hide from the immune system. Although S1P signaling is used by tumor cells for their benefit, none of the S1P-related receptors and enzymes are proto-oncogenes except for S1P2, which is involved in GCB-DLBCL lymphoma development when loss-of-function-mutations occur. Because of the tumor-promoting role of S1P signaling, agonists and antagonists for S1P receptors, inhibitors of SphKs, and S1P-blocking substances are promising candidates for cancer treatment.
Acknowledgments
Funding: The authors would like to thank the Federal Ministry of Education and Research (BMBF), Germany, FKZ 01E01502, and the German Research Foundation (DFG), GRK 1715 for their support.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Lysophospholipids on Immunity and Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.09.01). The series “Lysophospholipids on Immunity and Cancer” was commissioned by the editorial office without any funding or sponsorship. MHG served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allende ML, Sasaki T, Kawai H, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 2004;279:52487-92. [PubMed]
- Kharel Y, Lee S, Snyder AH, et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem 2005;280:36865-72. [PubMed]
- Zemann B, Kinzel B, Muller M, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 2006;107:1454-8. [PubMed]
- Mizugishi K, Yamashita T, Olivera A, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 2005;25:11113-21. [PubMed]
- An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem 2000;275:288-96. [PubMed]
- Blom T, Bergelin N, Meinander A, et al. An autocrine sphingosine-1-phosphate signaling loop enhances NF-kappaB-activation and survival. BMC Cell Biol 2010;11:45. [PubMed]
- Donati C, Cencetti F, Nincheri P, et al. Sphingosine 1-phosphate mediates proliferation and survival of mesoangioblasts. Stem Cells 2007;25:1713-9. [PubMed]
- Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci 1997;17:6952-60. [PubMed]
- Hamada K, Nakamura H, Oda T, et al. Involvement of Mac-1-mediated adherence and sphingosine 1-phosphate in survival of phorbol ester-treated U937 cells. Biochem Biophys Res Commun 1998;244:745-50. [PubMed]
- Karliner JS, Honbo N, Summers K, et al. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol 2001;33:1713-7. [PubMed]
- Kim DS, Hwang ES, Lee JE, et al. Sphingosine-1-phosphate promotes mouse melanocyte survival via ERK and Akt activation. Cell Signal 2003;15:919-26. [PubMed]
- Riccitelli E, Giussani P, Di Vito C, et al. Extracellular sphingosine-1-phosphate: a novel actor in human glioblastoma stem cell survival. PLoS One 2013;8:e68229 [PubMed]
- Rodriguez AM, Graef AJ, LeVine DN, et al. Association of Sphingosine-1-phosphate (S1P)/S1P Receptor-1 Pathway with Cell Proliferation and Survival in Canine Hemangiosarcoma. J Vet Intern Med 2015;29:1088-97. [PubMed]
- Weigert A, Johann AM, von Knethen A, et al. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood 2006;108:1635-42. [PubMed]
- Zhang J, Honbo N, Goetzl EJ, et al. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 2007;293:H3150-8. [PubMed]
- Zhao Y, Kalari SK, Usatyuk PV, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem 2007;282:14165-77. [PubMed]
- Le Stunff H, Galve-Roperh I, Peterson C, et al. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol 2002;158:1039-49. [PubMed]
- Ogawa C, Kihara A, Gokoh M, et al. Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2. J Biol Chem 2003;278:1268-72. [PubMed]
- Andréani P, Gräler MH. Comparative quantification of sphingolipids and analogs in biological samples by high-performance liquid chromatography after chloroform extraction. Anal Biochem 2006;358:239-46. [PubMed]
- Saba JD, Nara F, Bielawska A, et al. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J Biol Chem 1997;272:26087-90. [PubMed]
- Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005;309:1735-9. [PubMed]
- Weber C, Krueger A, Münk A, et al. Discontinued postnatal thymocyte development in sphingosine 1-phosphate-lyase-deficient mice. J Immunol 2009;183:4292-301. [PubMed]
- Zhou J, Saba JD. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem Biophys Res Commun 1998;242:502-7. [PubMed]
- Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996;381:800-3. [PubMed]
- Lavieu G, Scarlatti F, Sala G, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem 2006;281:8518-27. [PubMed]
- Sensken SC, Bode C, Nagarajan M, et al. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J Immunol 2010;184:4133-42. [PubMed]
- Johnson KR, Becker KP, Facchinetti MM, et al. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA). J Biol Chem 2002;277:35257-62. [PubMed]
- Fukuhara S, Simmons S, Kawamura S, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest 2012;122:1416-26. [PubMed]
- Kawahara A, Nishi T, Hisano Y, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 2009;323:524-7. [PubMed]
- An S, Bleu T, Huang W, et al. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett 1997;417:279-82. [PubMed]
- Im DS, Heise CE, Ancellin N, et al. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem 2000;275:14281-6. [PubMed]
- Lee MJ, Van Brocklyn JR, Thangada S, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 1998;279:1552-5. [PubMed]
- Van Brocklyn JR, Gräler MH, Bernhardt G, et al. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood 2000;95:2624-9. [PubMed]
- Bektas M, Allende ML, Lee BG, et al. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J Biol Chem 2010;285:10880-9. [PubMed]
- Billich A, Baumruker T, Beerli C, et al. Partial deficiency of sphingosine-1-phosphate lyase confers protection in experimental autoimmune encephalomyelitis. PLoS One 2013;8:e59630 [PubMed]
- Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J 2004;18:551-3. [PubMed]
- Lo CG, Xu Y, Proia RL, et al. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med 2005;201:291-301. [PubMed]
- Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355-60. [PubMed]
- Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007;316:295-8. [PubMed]
- Pham TH, Okada T, Matloubian M, et al. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity 2008;28:122-33. [PubMed]
- Vogel P, Donoviel MS, Read R, et al. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One 2009;4:e4112 [PubMed]
- Camerer E, Regard JB, Cornelissen I, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 2009;119:1871-9. [PubMed]
- Garcia JG, Liu F, Verin AD, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689-701. [PubMed]
- Lee OH, Kim YM, Lee YM, et al. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun 1999;264:743-50. [PubMed]
- Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006;9:225-38. [PubMed]
- Lee H, Deng J, Kujawski M, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 2010;16:1421-8. [PubMed]
- Gatfield J, Monnier L, Studer R, et al. Sphingosine-1-phosphate (S1P) displays sustained S1P1 receptor agonism and signaling through S1P lyase-dependent receptor recycling. Cell Signal 2014;26:1576-88. [PubMed]
- Moyer MP. Tumor cell culture. Methods Enzymol 1995;254:153-65. [PubMed]
- Bazzi MD, Nelsestuen GL. Mechanism of protein kinase C inhibition by sphingosine. Biochem Biophys Res Commun 1987;146:203-7. [PubMed]
- Hannun YA, Bell RM. Regulation of protein kinase C by sphingosine and lysosphingolipids. Clin Chim Acta 1989;185:333-45. [PubMed]
- Johnson JA, Clark RB. Multiple non-specific effects of sphingosine on adenylate cyclase and cyclic AMP accumulation in S49 lymphoma cells preclude its use as a specific inhibitor of protein kinase C. Biochem J 1990;268:507-11. [PubMed]
- Mullmann TJ, Siegel MI, Egan RW, et al. Sphingosine inhibits phosphatidate phosphohydrolase in human neutrophils by a protein kinase C-independent mechanism. J Biol Chem 1991;266:2013-6. [PubMed]
- Goldkorn T, Dressler KA, Muindi J, et al. Ceramide stimulates epidermal growth factor receptor phosphorylation in A431 human epidermoid carcinoma cells. Evidence that ceramide may mediate sphingosine action. J Biol Chem 1991;266:16092-7. [PubMed]
- Sadahira Y, Ruan F, Hakomori S, et al. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A 1992;89:9686-90. [PubMed]
- Bode C, Berlin M, Röstel F, et al. Evaluating sphingosine and its analogues as potential alternatives for aggressive lymphoma treatment. Cell Physiol Biochem 2014;34:1686-700. [PubMed]
- Haimovitz-Friedman A, Kan CC, Ehleiter D, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med 1994;180:525-35. [PubMed]
- Hannun YA, Obeid LM. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci 1995;20:73-7. [PubMed]
- Lavieu G, Scarlatti F, Sala G, et al. Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy 2007;3:45-7. [PubMed]
- Zhang Y, Wang Y, Wan Z, et al. Sphingosine kinase 1 and cancer: a systematic review and meta-analysis. PLoS One 2014;9:e90362 [PubMed]
- Rex K, Jeffries S, Brown ML, et al. Sphingosine kinase activity is not required for tumor cell viability. PLoS One 2013;8:e68328 [PubMed]
- Rutherford C, Childs S, Ohotski J, et al. Regulation of cell survival by sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent suppression of Bim and PI-3-kinase/protein kinase C-mediated upregulation of Mcl-1. Cell Death Dis 2013;4:e927 [PubMed]
- Shen H, Zhou E, Wei X, et al. High density lipoprotein promotes proliferation of adipose-derived stem cells via S1P1 receptor and Akt, ERK1/2 signal pathways. Stem Cell Res Ther 2015;6:95. [PubMed]
- Safarian F, Khallaghi B, Ahmadiani A, et al. Activation of S1P(1) receptor regulates PI3K/Akt/FoxO3a pathway in response to oxidative stress in PC12 cells. J Mol Neurosci 2015;56:177-87. [PubMed]
- Nakahara T, Iwase A, Nakamura T, et al. Sphingosine-1-phosphate inhibits H2O2-induced granulosa cell apoptosis via the PI3K/Akt signaling pathway. Fertil Steril 2012;98:1001-8.e1.
- Liu G, Burns S, Huang G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol 2009;10:769-77. [PubMed]
- Bergelin N, Lof C, Balthasar S, et al. S1P1 and VEGFR-2 form a signaling complex with extracellularly regulated kinase 1/2 and protein kinase C-alpha regulating ML-1 thyroid carcinoma cell migration. Endocrinology 2010;151:2994-3005. [PubMed]
- Li MH, Sanchez T, Yamase H, et al. S1P/S1P1 signaling stimulates cell migration and invasion in Wilms tumor. Cancer Lett 2009;276:171-9. [PubMed]
- Yuan LW, Liu DC, Yang ZL. Correlation of S1P1 and ERp29 expression to progression, metastasis, and poor prognosis of gallbladder adenocarcinoma. Hepatobiliary Pancreat Dis Int 2013;12:189-95. [PubMed]
- Green JA, Cyster JG. S1PR2 links germinal center confinement and growth regulation. Immunol Rev 2012;247:36-51. [PubMed]
- Green JA, Suzuki K, Cho B, et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol 2011;12:672-80. [PubMed]
- Cattoretti G, Mandelbaum J, Lee N, et al. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res 2009;69:8686-92. [PubMed]
- Morin RD, Mungall K, Pleasance E, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood 2013;122:1256-65. [PubMed]
- Yonesu K, Kawase Y, Inoue T, et al. Involvement of sphingosine-1-phosphate and S1P1 in angiogenesis: analyses using a new S1P1 antagonist of non-sphingosine-1-phosphate analog. Biochem Pharmacol 2009;77:1011-20. [PubMed]
- Schmid G, Guba M, Ischenko I, et al. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J Cell Biochem 2007;101:259-70. [PubMed]
- LaMontagne K, Littlewood-Evans A, Schnell C, et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res 2006;66:221-31. [PubMed]
- Chae SS, Paik JH, Furneaux H, et al. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest 2004;114:1082-9. [PubMed]
- Morii T, Weissbach L. Sphingosine 1-phosphate and cell migration: resistance to angiogenesis inhibitors. Biochem Biophys Res Commun 2003;310:884-8. [PubMed]
- Du W, Takuwa N, Yoshioka K, et al. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res 2010;70:772-81. [PubMed]
- Takabe K, Paugh SW, Milstien S, et al. "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 2008;60:181-95. [PubMed]
- Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J Lipid Res 2014;55:1839-46. [PubMed]
- Linton KJ. Structure and function of ABC transporters. Physiology (Bethesda) 2007;22:122-30. [PubMed]
- Ihlefeld K, Vienken H, Claas RF, et al. Upregulation of ABC transporters contributes to chemoresistance of sphingosine 1-phosphate lyase-deficient fibroblasts. J Lipid Res 2015;56:60-9. [PubMed]
- Mitra P, Oskeritzian CA, Payne SG, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A 2006;103:16394-9. [PubMed]
- Nieuwenhuis B, Lüth A, Chun J, et al. Involvement of the ABC-transporter ABCC1 and the sphingosine 1-phosphate receptor subtype S1P(3) in the cytoprotection of human fibroblasts by the glucocorticoid dexamethasone. J Mol Med (Berl) 2009;87:645-57. [PubMed]
- Takabe K, Kim RH, Allegood JC, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem 2010;285:10477-86. [PubMed]
- Tanfin Z, Serrano-Sanchez M, Leiber D. ATP-binding cassette ABCC1 is involved in the release of sphingosine 1-phosphate from rat uterine leiomyoma ELT3 cells and late pregnant rat myometrium. Cell Signal 2011;23:1997-2004. [PubMed]
- Bode C, Sensken SC, Peest U, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem 2010;109:1232-43. [PubMed]
- Lee YM, Venkataraman K, Hwang SI, et al. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC). Prostaglandins Other Lipid Mediat 2007;84:154-62. [PubMed]
- Laurenzana A, Cencetti F, Serrati S, et al. Endothelial sphingosine kinase/SPNS2 axis is critical for vessel-like formation by human mesoangioblasts. J Mol Med (Berl) 2015;93:1145-57. [PubMed]
- Bradley E, Dasgupta S, Jiang X, et al. Critical role of Spns2, a sphingosine-1-phosphate transporter, in lung cancer cell survival and migration. PLoS One 2014;9:e110119 [PubMed]
- O'Brien N, Jones ST, Williams DG, et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res 2009;50:2245-57. [PubMed]
- Purschke WG, Hoehlig K, Buchner K, et al. Identification and characterization of a mirror-image oligonucleotide that binds and neutralizes sphingosine 1-phosphate, a central mediator of angiogenesis. Biochem J 2014;462:153-62. [PubMed]
- Ader I, Gstalder C, Bouquerel P, et al. Neutralizing S1P inhibits intratumoral hypoxia, induces vascular remodelling and sensitizes to chemotherapy in prostate cancer. Oncotarget 2015;6:13803-21. [PubMed]
- Berdyshev EV, Gorshkova I, Usatyuk P, et al. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS One 2011;6:e16571 [PubMed]
- Brizuela L, Martin C, Jeannot P, et al. Osteoblast-derived sphingosine 1-phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis-derived prostate cancer cells. Mol Oncol 2014;8:1181-95. [PubMed]
- Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer 2006;95:1131-5. [PubMed]
- Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol 2011;162:1225-38. [PubMed]
- Schneider G, Bryndza E, Abdel-Latif A, et al. Bioactive lipids S1P and C1P are prometastatic factors in human rhabdomyosarcoma, and their tissue levels increase in response to radio/chemotherapy. Mol Cancer Res 2013;11:793-807. [PubMed]
- Zhang L, Wang X, Bullock AJ, et al. Anti-S1P Antibody as a Novel Therapeutic Strategy for VEGFR TKI-Resistant Renal Cancer. Clin Cancer Res 2015;21:1925-34. [PubMed]
- Antoon JW, White MD, Burow ME, et al. Dual inhibition of sphingosine kinase isoforms ablates TNF-induced drug resistance. Oncol Rep 2012;27:1779-86. [PubMed]
- Baran Y, Salas A, Senkal CE, et al. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem 2007;282:10922-34. [PubMed]
- Brizuela L, Ader I, Mazerolles C, et al. First evidence of sphingosine 1-phosphate lyase protein expression and activity downregulation in human neoplasm: implication for resistance to therapeutics in prostate cancer. Mol Cancer Ther 2012;11:1841-51. [PubMed]
- Colié S, Van Veldhoven PP, Kedjouar B, et al. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res 2009;69:9346-53. [PubMed]
- Gao H, Deng L. Sphingosine kinase-1 activation causes acquired resistance against Sunitinib in renal cell carcinoma cells. Cell Biochem Biophys 2014;68:419-25. [PubMed]
- Guan H, Song L, Cai J, et al. Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to apoptosis resistance in glioma cells. PLoS One 2011;6:e19946 [PubMed]
- Illuzzi G, Bernacchioni C, Aureli M, et al. Sphingosine kinase mediates resistance to the synthetic retinoid N-(4-hydroxyphenyl)retinamide in human ovarian cancer cells. J Biol Chem 2010;285:18594-602. [PubMed]
- Rosa R, Marciano R, Malapelle U, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res 2013;19:138-47. [PubMed]
- Salas A, Ponnusamy S, Senkal CE, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 2011;117:5941-52. [PubMed]
- Sharma N, He Q, Sharma RP. Sphingosine kinase activity confers resistance to apoptosis by fumonisin B1 in human embryonic kidney (HEK-293) cells. Chem Biol Interact 2004;151:33-42. [PubMed]
- Song L, Xiong H, Li J, et al. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-kappaB pathway in human non-small cell lung cancer. Clin Cancer Res 2011;17:1839-49. [PubMed]
- Watson C, Long JS, Orange C, et al. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol 2010;177:2205-15. [PubMed]
- Liu Y, Deng J, Wang L, et al. S1PR1 is an effective target to block STAT3 signaling in activated B cell-like diffuse large B-cell lymphoma. Blood 2012;120:1458-65. [PubMed]
- Liu G, Yang K, Burns S, et al. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol 2010;11:1047-56. [PubMed]
- Priceman SJ, Shen S, Wang L, et al. S1PR1 is crucial for accumulation of regulatory T cells in tumors via STAT3. Cell Rep 2014;6:992-9. [PubMed]


