
Stereotactic body radiation therapy in lung
Introduction
Treatment of choice for stage I (T1-T2N0) non-small-cell lung cancer (NSCLC) is surgery. But many patients are unable to tolerate the resection due to poor pulmonary reserve or medically inoperable due to multiple comorbid conditions. Five-year overall survival for untreated patients with stage I NSCLC is 5% and median survival 9 months (13 months for T1), as reported by Raz et al. based on California cancer registry (1).
Primary radiation therapy is considered to be reasonable therapy, for non-surgical early-stage NSCLC with reported 5-year survival rates ranging from 10% to 30% (2).
A review of 156 medically inoperable patients with stage I NSCLC at Duke University between 1980 and 1995 demonstrated a 5-year, cause-specific survival rate of 32% with RT alone. Improved survival was significantly correlated with achieving local control and approached significance for higher RT doses (3).
The standard approach involves administering an approximate dose of 4,500 to 6,600 cGy in fractions of 180 to 200 cGy. Historically, RT fields for early-stage NSCLC encompassed the primary tumor and regional lymphatics in the ipsilateral hilum and mediastinum. This “prophylactic” treatment was based on the identified risk of occult nodal involvement from surgical series ranging up to 20%, and surgical data indicating better control with more extensive resections (4).
However, large RT fields are potentially poorly tolerated in this population of patients with limited pulmonary reserves. More recent retrospective experiences have demonstrated similar survival results with fields limited to the primary tumor or gross disease alone, compared to fields including prophylactic treatment to lymph node chains (5,6).
Several studies reported safety and feasibility of dose escalation using 3D conformal radiation therapy to the gross disease alone omitting elective nodal irradiation was studied (7,8).
In a report from the Netherlands, limited “postage-stamp” fields were treated using hypo fractionated RT (i.e., 4,800 cGy in 400 cGy fractions) with reported 3-year overall and disease-specific survival rates of 42% and 76%, respectively (9).
The only dose finding study of stereotactic body radiation therapy (SBRT) for lung tumors was reported by Timmerman et al. from Indiana. They conducted a phase I study of dose escalation of a 3 fractions regiment, starting with 8 Gy ×3, and escalating to 10, 12, 14, 16, 18, 20 and 22 Gy ×3 fractions, in patients with potentially resectable NSCLC but who were not surgical candidates for medical reasons (“medically inoperable”). Doses were calculated without correction for tissue inhomogeneity. Patients were enrolled into three separate dose escalation groups based on tumor size. While dose-limiting toxicity (DLT) was observed in one or two patients at several dose levels, the protocol defined maximum tolerated dose (MTD) was only observed in patients with large T2 tumors (5-7 cm in size) at 22 Gy ×3. In other tumor size groups, dose escalation was stopped prior to reaching the MTD (20-22 Gy ×3). Greater than 90% primary tumor control was observed with 20 Gy ×3; this total dose of 60 Gy corresponds to a biologically equivalent dose (BED) (if expressed in 2 Gy/fraction) of 180 Gy if using the formula BED = nd (1+ d/alpha/beta), where n = number of fractions; d = dose per fraction; and alpha/beta =10 for acute reacting tissue), although it is not clear how applicable this conversion is to highly hypofractionated treatments (10).
In a subsequent single institution phase II study of this SBRT regiment, Timmerman and colleagues treated 70 patients with early stage (T1-2, N0) inoperable NSCLC with 60 Gy in 3 fractions for T1 and 66 Gy in 3 fractions for T2.14 That study allowed enrollment of patients with tumors located anywhere within the lung, and confirmed high rates of primary tumor control: 95% at 2 years. After median follow up of 17.5 months, three patients demonstrated a local recurrence. The study was particularly instructive in terms of local toxicity: eight patients were deemed by the data safety monitoring board to have grade 3 or 4 adverse events resulting from SBRT; the adverse events were primarily respiratory (decline in pulmonary function, pneumonia, pleural effusion, apnea) and/or skin reaction; they occurred a median of 7.6 months after completion of SBRT. Six patients may potentially have had grade 5 (i.e., fatal) toxicity. In five patients, these grade 5 adverse events were respiratory: one fatal hemoptysis (associated with a local recurrence) and four infectious pneumonias; the sixth patient died of complications from a pericardial effusion. These deaths occurred a median of 10.4 months after SBRT (range, 0.6-19.5 months). Tumor location was a strong predictor of toxicity, with hilar or pericentral tumors showing an 11-fold increased risk in grade 3-5 adverse events when compared to more peripheral tumors (P=0.004). Two-year freedom from severe adverse events was 54% for these central tumors, as compared to 83% for the peripheral tumors, defined as outside the “zone of the proximal bronchial tree”, which is a 2 cm radius around the main tracheo-bronchial tree: trachea; left and right main stem bronchi; right upper, middle, and lower lobe bronchus; and left upper, lingular, and lower lobe bronchus. The only other variable that was a predictor of toxicity, although not as strong as tumor location, was the size of gross tumor volume (GTV), with >10 cc tumors showing greater toxicity than smaller GTVs (11).
On the basis of these two studies, 60 Gy in 3 fractions was chosen as the dose for the RTOG-led phase II multicenter study, RTOG 0236, but patients with tumors within the above-described zone of proximal bronchial tree were excluded from the study. As in the prior phase I and II studies, the doses were calculated without correction for tissue inhomogeneity.
Five-year results of this study were presented at ASTRO 2014. In the of 55 evaluable patients, Primary recurrence was 7% (4/55), lobar recurrence 20% (9/55), loco-regional recurrence 38% (7/55-Nodal + adjacent organs), Disseminated failure entire lung: 31% (15/55). Disease free survival 26%, Overall survival 40% and Median survival were 4 years. Pulmonary toxicity observed was grade 3 in 27% (15/55), grade 4 in 3.6% (2/55) and no grade 5.
Radiobiology of SBRT
Radiation death is defined as loss of reproductive integrity of the cell when exposed to radiation. Traditionally it was explained by damage of DNA with radiation. Biologically effective dose (BED) based on the linear-quadratic (LQ) model is as follows:
BED =
In this calculation, n equals the number of fractions and d equals the fraction size. The α component represents the linear portion of the cell survival curve, where a single radiation event (DNA double-strand break) causes cell death. The β component represents the quadratic portion of the cell survival curve, where cell death results from at least two double-strand breaks (12).
But at hypo fractionated regimens that were used in SBRT vascular effects due to endothelial apoptosis appears to play a major role. Endothelial cells upon exposure to high dose of radiation (>10 Gy) acid sphingomyelinase is translocated to the plasma membrane of endothelial cells where it plays a role in generating ceramide from sphingomyelin. Ceramide release leads to activation of the apoptotic protein BAX (13,14).
BAX is part of the Bcl-2 family of proteins and is important pro apoptotic regulator. Activation of BAX leads to the release of mitochondrial cytochrome c, which signifies commitment of the cell to apoptosis via intrinsic pathway (15). Endothelial apoptosis peaks within 6 hours after radiation and causes micro vascular dysfunction and hence acutely disrupts tumor perfusion (16).
SBRT in metastatic setting
Rusthoven et al., studied patterns of failure after SBRT following first line systemic therapy for metastatic lung cancer. Local failure was noted in 64%, distant only failure was noted in 9% and in 14% failed both local and distant together. SBRT dose range was from 36-60 Gy in 3 fractions. Time to first progression was 3 months in local only failure compared to 5.7 months in disatant failure (HR: 0.44; 95% CI: 0.22-0.90). This study suggests that SBRT could improve time to progression (17).
Another Ph II study by Iyengar et al., treated metastatic NSCLC with <6 metastatic lesions with SBRT after early failure of systemic therapy. Failure rate was 6.4% in the SBRT treated lesions. Majority of patients progressed in new distant sites. Median progression free survival was 14.7 months and overall survival was 20.4 months, which exceeded the historical controls (18).
These initial studies proved the benefit of aggressive local treatment in the oligometastatic setting and safety of treating the metastases with SBRT when the lesions are at least 5 cm apart.
At present NRG-BR001 studying the safety of SBRT in treating multiple metastases particularly >3 or 2 lesions separated by less than 5 cm.
Mediastinal staging
Accurate mediastinal staging is essential for the treatment planning of SBRT patients with NSCLC to ensure they do not have lymph node metastasis. In addition to a traditional mediastinoscopy noninvasive methods have been developed. These include Computed tomography (CT) scans, FDG PET scan and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and endobronchial ultrasound-guided fine needle aspiration (EBUS-FNA).
CT provides an excellent anatomic detail in mediastinal staging of NSCLC. However, approximately 40% of nodes reported as malignant by CT criteria are benign, and 20% reported as benign prove to be malignant (19). In patients with clinical stage I tumors, 5% to 15% will have positive lymph nodes at surgery (20). Dwamena et al. in a metaanalysis showed an average CT sensitivity of 60% and specificity of 77% for the detection of mediastinal nodal metastases (21). In 2003, another meta-analysis by Toloza et al. reported the pooled sensitivity and specificity for CT at 57% and 82%, respectively (22). The 2007 American College of Chest Physicians (ACCP) Evidence-Based Practice Guidelines reported 51% pooled sensitivity and 85% pooled specificity (23). Hence CT falls short in its ability to accurately stage the mediastinum.
The major benefit of fluorodeoxyglucose-PET scans in the lung cancer is its ability to provide functional information during the evaluation for intrathoracic and extrathoracic metastases. Numerous studies have demonstrated a higher sensitivity and specificity for PET than CT in the detection of malignant mediastinal nodes, with various meta-analyses reporting PET sensitivities of 74% to 85% and specificities of 85% to 92% (24,25). A high negative predictive value (NPV) of >90% in nodal staging has also been reported (26). Normal physiologic uptake and artifacts can lead to false-positive (FP) results. The ability of PET to resolve small hypermetabolic abnormalities in nodes is limited (27). Takamochi et al. studied PET limitations in nodal staging in NSCLC and reported low spatial resolution as a major causative factor for their 20% False negative rate (28). PET also could not identify small tumor foci ranging from 1 to 7.5 mm. A Cochrane data base (29) review of 45 studies concluded that sensitivity and specificity estimates for PET-CT positivity criterion were 77.4% (95% CI: 65.3-86.1) and 90.1% (95% CI: 85.3-93.5), respectively. They concluded that PET CT alone could not be used in mediastinal staging of lung cancer. Thus current imaging advancements have not, however, supplanted invasive staging (30,31).
EUS-FNA is generally regarded as a safe procedure. Contraindications are few, and include inability to tolerate conscious sedation, esophageal obstruction, and uncorrectable blood dyscrasia. Complications are rare and usually minor (32). Lymph nodes as small as 4 to 6 mm can be detected by EUS as long as they are in the vicinity of the esophagus and not obscured by tracheal air or intervening blood vessels. A recent review of 2,756 patients demonstrated overall median sensitivity of 89% and NPV of 91% (19). A meta-analysis in 2008 by Puli et al. reported that FNA raised the sensitivity of EUS in the diagnosis of mediastinal adenopathy from 85% to 88% and the specificity from 85% to 96% (33). Prenzel et al. (34) reported that lymph node size was not a reliable predictor of metastatic involvement; 44% of metastatic lymph nodes in NSCLC patients studied measured <1 cm in short axis, 77% of patients without nodal metastases had a lymph node >1 cm, and 12% of patients with nodal metastases had no nodes >10 mm. Sonographic characteristics of lymph nodes identified during EUS have also been studied. Features reported as predictive of malignancy include rounded contour, sharply circumscribed border, hypoechoic echogenicity, and >1 cm diameter. An increased number of these features has been associated with a higher likelihood that a particular lymph node is malignant (80% to 100%), with 25% of malignant nodes reportedly fulfilling all four conditions (35,36). Kramer and Groen (37) published a meta-analysis of 14 studies in 2003 and reported the sensitivity of EUS-FNA as 81% to 97% and the specificity as 83% to 100% for the diagnosis of posterior mediastinal lymphadenopathy. In 2007, Micames et al. published a meta-analysis of 18 studies and reported a pooled sensitivity of 83% and specificity of 97% (32). EUS-FNA is therefore been recommended for staging of the mediastinum when CT and PET do not show disease. Mediastinoscopy should only be performed for patient with a high probability of having nodal disease and the EUS-FNA was negative for malignancy.
Practical aspects of planning SBRT
SBRT typically refers to a radiation therapy technique in which an extracranial tumor receives high doses (7-30 Gy) of radiation following a hypofractionated prescription of 5 or less fractions. Provision of these high doses while also achieving normal tissue doses less than tolerance is characterized by tight conformation of the prescription dose to the target volume, steep gradient fall-off away from the target edge, and a high level of inhomogeneity of target dose. Due to the levels of conformity, inhomogeneity, and dose gradient fall-off, accurate tumor delineation, dose modeling, and treatment delivery are of extreme importance even compared to conventional intensity modulated radiation therapy (IMRT). These high standards of accuracy and precision for SBRT have led to much tighter tolerances when traditional QA tests are performed on treatment machines, treatment planning systems, and even patient plans, i.e., the guidance document published by the AAPM on QA of Linear accelerators where machines used to deliver SBRT are separated from those used for only conventional IMRT or 3D. In addition to the need for increased accuracy, proper and successful SBRT to the lung requires the consideration of another component which is delivery to a moving target. Consideration of the need for increased accuracy and breathing motion must occur at all steps in the radiation treatment planning and delivery process for SBRT Lung. What follows is a discussion of practical aspects of the aforementioned process (38-41).
Physics preparation
Prior to beginning a treatment technique, it must be commissioned by the physics staff. As small fields (i.e., <3 cm × 3 cm) techniques are to be used, this will likely include the acquisition of further beam data and characteristics that likely will not currently be included in the planning system. The treatment device used will need to be tuned and adjusted to meet stereotactic tolerances. Perhaps a totally new treatment device is to be used in which case this device (i.e., Cyberknife, Vero, ViewRay, etc.) will need to be commissioned for complete clinical use rather than simply for a given technique. Even among individual machines, accessories to be used in SBRT lung may differ such as stereotactic cones, multi-leaf collimator (MLC), or even micro-MLC and, therefore each must be commissioned before use. Motion management systems will also have to be tested and implemented properly. This work will require the physicist to be familiar with new and unconventional equipment even including the detectors used for data acquisition. The use of redundant equipment such as detectors is highly recommended so that clinical data obtained with each is corroborated by that obtained by the other (41,42). Proper procedures for this are extensive and require significant attention to detail, thus the full discussion of the topic is beyond the scope of this writing, however, SBRT commissioning processes have been described extensively in literature including a few American Association of Physicists in Medicine (AAPM) task group (TG) reports. Recommendations from those guidance documents and others should be understood and followed (39,41-43).
Simulation
Simulation of a patient to be treated with SBRT to the Lung basically involves two parts:
- Reproducible patient positioning and immobilization;
- Proper acquisition of patient data (i.e., imaging).
Patient setup and immobilization
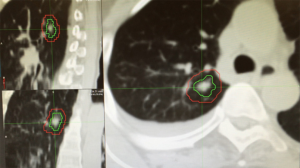
Patient setup and immobilization has come a long way since the introduction of 3D imaging and conventional IMRT. In order to provide consistent and reproducible setup, stereotactic body frames have been developed by a number of vendors. Many of the current generation of frames includes a fiducial-based localization system, however, most clinics avoid the use of such in body radiosurgery due to the availability of accurate image guidance and the inconsistency of tumor location in the body compared to coordinates based on external fiducials. These frames often consist of vacuum bags conformed to a large portion of the patient’s body with the added option of active breathing management to be discussed later (44). Despite improvements in setup and immobilization for use in SBRT, the need for image guidance has been shown (Figure 1) (45,46).

Acquisition of patient data
Imaging and motion management
Technically, proper tumor diagnosis and/or biopsy is a major part of this process; however, for the sake of this discussion, the focus will primarily be on the imaging portion of this step. Currently, CT is the modality of choice for treatment planning for lung SBRT. This is primarily due to the feasibility of reasonably accurate dose calculations based on the relationship of electron density and CT number which allows for proper consideration of tissue heterogeneity and radiation transport. All simulations of SBRT lung patients will utilize CT and then will take it a step further with the use of 4D CT. 4D CT combines the capture of a representation of the patient’s breathing cycle with simultaneous CT imaging during the breathing motion. The patient breathing is graphed as a sinusoidal curve and during reconstruction the CT images are then organized based on the time point in the breathing cycle at which they were taken. Theoretically, each image would be mapped directly to the exact point in the respiratory cycle that it was taken and “binned” into a CT dataset with all other CT images scanned at that time point and each position. However, since there are infinite arbitrary time points in the cycle, the result would be CT datasets with limited numbers of images that would not represent the entire area scanned for all time points. For practical implementation, the respiratory cycle is divided into “phases” based on when in the cycle it occurs and each phase represents a range of time points in the cycle. Then, each CT image for a given slice position and given time point in a “phase” are sorted with all other CT images that occur at different slice positions but within the same “phase” of the respiratory cycle. Using the resulting datasets(typically 10 phases), one can hold the slice position constant, but rotate through the different phase datasets in order of their position in time on the respiratory cycle and the motion of the anatomy at that slice position should be represented as a “video”. The aforementioned method represents phase binning and is the most commonly utilized 4D reconstruction method; however, amplitude binning is also an option utilized based on needs, raw data, and desired results. Also, typically, prospective binning is performed, but strategies exist for retrospective binning when desired results are not achieved by the latter (47-49).
Vendors provide different techniques for capturing the breathing cycle which utilize different forms of “surrogates” for respiration. Varian’s RPM utilizes a camera system to watch external fiducials placed on the abdomen. The C-RAD Sentinel system implements a scanning laser over the abdominal surface. Philips interfaces with a bellows system around the abdomen that monitors air flow dependent on the position of the abdominal surface. Even the Microsoft KINECT has been tested for use in the acquisition of the respiratory cycle. Regardless of the system utilized, the desired endpoint is the same and certain uncertainties exist which should be taken into account during the remaining treatment planning process. Some of these uncertainties have been described as inaccurate binning of CT images into their respective phase, non-correlation of a respiratory surrogate to actual tumor motion, and non-reproducibility of respiratory cycle throughout patient treatment. These uncertainties should be accounted for during the treatment planning and delivery process (40,50,51).
In addition to the 4D phase datasets, the data obtained from 4D scanning can also be reconstructed into intensity projection datasets. Maximum intensity projection (MIP) datasets are represented by each voxel being assigned its maximum CT number that occurred during the 4D cycle. Average intensity projection (Ave-IP) and minimum intensity projection (mini-IP) follow the same logic, but with the average and minimum CT numbers respectively. Theoretically representing the maximum tumor motion, the MIP comes into play as a useful single shot representation of the motion displayed by the 4D phases. The Ave-IP often comes into play when considering the optimal image for dose calculation. Mini-IP is not used very often in regards to lung, but does offer value in radiation with tumors in the abdomen, such as liver or pancreas (40,52-54).
Rather than simply acquiring the full potential tumor motion in an image, one may also take steps to actively reduce target motion before imaging it. Many types of active motion control exist with the simplest being to image during a breath hold at a particular time point in the respiratory cycle (typically full inspiration or full exhalation) with the intent of treating with this same breath hold status. A few systems have been designed that can assist the patient in reproducing the same breath hold each time while also communicating with the radiation oncology staff about the actual status of the patient’s breathing. Another technique for motion reduction is to apply some type of abdominal compression. One form of this involves placing specially designed plastic wrap over the patient in their vacuum bag and then evacuating the air out from underneath it. A more rigid type of compression exists in the form of a frame that is placed over the patient’s abdomen where a flat pad can be screwed down to apply pressure to the patient’s upper abdomen until the desired tumor motion is achieved when reviewed with imaging. Another type of active motion management is referred to as respiratory gating. Implementation of this technique will involve physician review of the 4D CT. He or she will decide which of the phases contain the target within an acceptable margin and the target delineation and treatment will be adjusted to only treat the outlined area during those chosen phases. Many have begun utilizing the placement of radiopaque fiducials in or near the tumor. This is typically done by the surgeon and usually greater than three days before the patient’s scheduled radiation oncology simulation and assists in target identification and localization throughout the entire simulation and treatment process. Often, multiple types of motion management are used in tandem during the treatment process (40,41,43).
Imaging and target identification
In addition to motion management, one must consider the proper identification of the proposed target. Lung tumors, especially in the typical SBRT lung patient, can be shrouded by non-cancerous tissue that may obscure or even masquerade as the tumor itself. This can be especially problematic with tumors located near the diaphragm or in the presence of heavy atelectasis. The most common method of alleviating this issue is currently with the utilization of positron emission tomography (PET) often in conjunction with an anatomical CT (PET/CT). PET increases the specificity of imaging of malignant tissue and when fused with the planning images, can assist in accurate delineation of the tissues to be treated. Ideally, this PET image would be performed close to the simulation date and in the proposed treatment position to reduce the fusion uncertainty. This fusion can be performed rigidly or deformably using multiple types of software including most modern treatment planning systems (55,56). Another option to assist in target identification is the placement of radiopaque fiducials as mentioned above. The use of fiducials assists in target identification throughout the entire simulation and treatment delivery process (57,58).
Practical simulation considerations
As stated previously, the simulation should result in reproducible patient positioning and immobilization as well as proper acquisition of patient data for planning and treatment. For reproducibility, consideration should be given to items such as patient comfort, habitus, and mental status. Sometimes medication can be used to assist a patient in relaxation both at simulation and treatment. Ideally, a patient would be setup in such a manner so that pre-treatment corrections could be maximally applied (robotic couches offer 6 degrees of correction and submillimeter corrections opposed to traditional treatment couches with only 3 degrees and subcentimeter corrections) however this may require a frame with infrared markers which will not fit over patients of a given habitus. Also, in some cases, the desired patient position may not be easily achievable due to patient’s historical injuries or such and the simulation technique may need to be adapted. In general, though, patients should be positioned head first and supine with their arms up inside their immobilization device. CT imaging should achieve ≤3 mm slice thickness (one could optionally use variable slice thickness on some scanners to scan thin slices in and near the tumor and thicker as you get away from it) and should cover all normal tissues of interest as accurate dose volume histogram (DVH) data will be necessary on these structures. Margin well above and below the area to be treated will be necessary for accurate dose calculation and also due to the probable use of noncoplanar beam angles (39,40).
Patient data that is also of interest during SBRT lung planning is the clearance distance between the gantry and the patient when various gantry, collimator, and couch positions are utilized. The acquisition of this data is usually performed in three basic ways. The first method is to simply scan a larger portion of the patient during simulation so that collisions can be anticipated virtually and avoided. A second method is to take the patient and their immobilization devices to the treatment room after simulation and perform a comprehensive dry run positioning the gantry, couch, and collimator at various places with the patient aligned roughly at isocenter. The third method basically ignores this possibility (not completely as the planner still tries to avoid collision) and a treatment dry run is performed before the first fraction. If a collision is discovered, then the plan is quickly adaptively planned to avoid the collision but still achieve the planning goals.
Treatment planning
The treatment planning process, in general, includes several steps such as delineation of target and normal tissue volumes, determination of prescription and fractionation schedule, and calculation and optimization of the dose distribution. This process has several additional considerations (some discussed above) when compared to conventional fractionation or non-lung treatments. Several Radiation Therapy Oncology Group (RTOG) trials exist that provide guidance and the opportunity for consistency in performance of the treatment planning process.
Contouring
Care should be taken to follow International Commission on Radiation Units (ICRU) and Measurements guidelines on the definition of target volumes. The GTV is delineated using a combination of what is visible on CT and PET, implanted fiducials, and clinical experience on one static CT image. For SBRT lung, the clinical target volume (CTV) is equal to the GTV. At this stage, the GTV is then expanded to the internal target volume (ITV) so that the ITV includes the GTV at all stages of the respiratory cycle. If the treatment utilizes respiratory gating, the ITV will only include the GTV on the phases to be included in the actual treatment delivery. Once the ITV has been created, it can then be expanded to create the planning target volume (PTV) using a geometrical expansion to account for setup uncertainty. RTOG protocols recommend a 5 mm expansion; however, one could justify a smaller number with high confidence in tumor localization. Normal tissues can be contoured according to RTOG guidelines. Typical evaluation structures for use during plan analysis are the body minus the PTV and the body minus the PTV with a 2 cm margin. A copy of the PTV may also be created to allow for volumetric control of the block margin around the PTV for better conformity (Figure 2) (59).
Dose prescription
This decision is made by the treating physician who may follow various protocols and guidelines that have been published. Typically, single fraction, high dose regimens are reserved for peripheral floating tumors that are “far” away from the mediastinum. Some people use the bronchial tree plus 2 cm in order to gauge whether a tumor is peripheral or central. Central lesions or those where rib fracture are a consideration are typically treated with more reserved fractionations in 3-5 fractions. Often when evaluating dose regimens, the LQ model can be used to calculate BED. Studies have shown that when BED >100 Gy, local control and survival significantly improves. Further discussion of the LQ model and its use in SBRT lung can be found elsewhere. One should note that the indiscriminate use of this BED model is not recommended as the LQ model is an approximation and use with heavy hypo-fractionation is not yet verified and the need for improvements on the model for use with SBRT have been indicated by some (60-62).
Dose calculation and optimization
General
Regardless of technique, there are certain considerations during the dose calculation and optimization phase of the treatment planning process. Typically, one must be prepared to use multiple beams or arcs and also that these beams or arcs will need to approach the patient from a noncoplanar direction. Energy selection >6 MV is highly discouraged to avoid the excessive lateral scatter that occurs in a low density medium such as lung. Due to the high gradients (possibly about 10-12% per mm) expected and encountered in this type of plan, the dose calculation grid must be set with a high enough resolution that the distribution is accurately characterized. For the sake of efficiency, initial planning can be performed at a lower resolution before changing it for the final stages of dose calculation and optimization. Quantitatively, TG 101 of the AAPM recommends grid spacing of ≤2 mm and strongly discourage grid spacing >3 mm. In addition to grid spacing, an appropriate algorithm must be selected that correctly handles lateral electron scattering in addition to the presence of heterogeneities and their interfaces. Most consider convolution-superposition algorithms a necessity and recommend Monte Carlo when available. Though not universally applied, many institutions take precautions to avoid calculating dose to “normal” lung when the goal is to treat “solid” tumor. These methods often include either using the Ave-IP for dose calculation or overriding the ITV to tissue density before dose calculation (39,40,52,53).
Regardless of planning technique, plans should be evaluated consistently using certain metrics. Typically, 100% of the prescription dose should cover 95% of the PTV and 90% of the prescription dose should cover 99% of the PTV (D95 =100%, D99 =90%). A conformity index should be used to ensure that only the PTV receives the prescription. Though inhomogeneity of dose is expected in SBRT lung, a homogeneity index should be used to govern that the level stays within a reasonable range such as that suggested by RTOG. A gradient index monitors that the desired gradient is achieved outside of the PTV to spare normal tissue. Various versions of these indices have been proposed. It should be noted that the values expected for the indices discussed above will differ depending on the exact treatment machine, accessories, and technique used in the treatment. The amount tissue outside of the PTV exposed to above prescription level dose should also be evaluated. Of course, dose to normal structures should also be evaluated. Constraints for all of the above have been listed in the various RTOG trial documents and mostly unvalidated normal tissue constraints have been published (Table 1) (63).
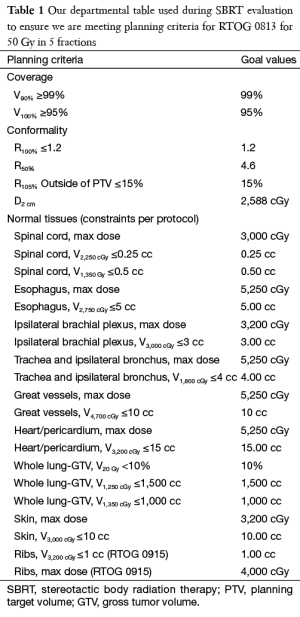
Full table
Prior to treatment of the patient on the machine and just as with any complex mode of radiation delivery, each patient’s treatment plan must undergo quality assurance on the treatment machine to ensure machine capabilities, no dose calculation mistakes, and proper electronic transmission of treatment parameters to the treatment machine. Various methods of this process have been described and are offered by many different vendors. Physicists should put for significant effort to not only understand their QA devices and methods, but also to establish stringent enough tolerances for the pass or fail of each plan as typical tolerances for conventional IMRT may not be acceptable. As with acquiring commissioning data, the use of multiple systems for corroboration is highly encouraged.
3D static fields
This technique is usually marked by 8-15 static fields directed at the PTV. Beams are arranged around the PTV in 20-40 gantry intervals typically avoiding the contralateral lung. A normalization point is placed at the center of mass of the PTV and the prescription is normalized to 60-90% at this point. With certain machines and accessories, it may be necessary to use more than 1 isocenter in order to achieve coverage or the technique may be nonisocentric in order to achieve coverage. Little or no block margin around the PTV is applied per RTOG protocols; however, best results are achieved when methods are utilized to create a variable margin around the PTV (if using MLC or non-static collimation such as cones). Typically, this means a positive margin of a few mm where the block edge intersects with a large amount of lung and a negative margin when the block edge may intersect or be near tissue density areas such as the chest wall or mediastinum. In some cases, the block may need to be adjusted to ensure that nearby normal tissues are appropriately spared. During plan optimization, multiple plan characteristics can be adjusted such as gantry, collimator, and couch positions, block margins, and prescription normalization percentage. Of course, what can be adjusted and how much is dependent on the machine and accessories in use.
3D conformal arcs
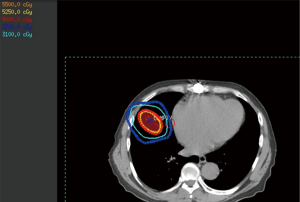
This technique involves one or more arcs during which either the isocenter is placed in the target so that the beam is always directed towards the target or the collimating device will direct the beam towards the target during the arc rotation. This technique is often optimized similarly to that of 3D static; however, it has certain tradeoffs when compared to it. It is often more difficult to achieve the same gradient with arcs, though the delivery time will be much shorter. In some cases, a hybrid plan involving 1-3 arcs and a noncoplanar static field or two will achieve planning objectives while sparing the efficiency (Figure 3).
IMRT static fields
Similarly to the relationship between conventional 3D and conventional IMRT, inverse optimization produces a treatment plan that meets the discussed goals potentially in a more efficient manner. Applied optimization objectives will be different with SBRT Lung as homogeneity within the PTV is not as important and a steep dose gradient is desired regardless of whether critical normal tissues are nearby. It should be mentioned that some institutions shy away from IMRT for SBRT lung due to the possibility of a large interplay effect within so few fractions. Some mitigate this issue with the use of gating and/or fiducial tracking. It should be noted that some studies have also found that this effect averages out over the total treatment, though the question remains whether the fractional dose is just as important as the total dose in SBRT lung. Regardless of feelings on the possible interplay effect, studies have shown that IMRT typically achieves better normal tissue sparing but less steep of a gradient when compared to 3D techniques and so may not be appropriate on a regular basis as this effect seems to magnify as target volumes become smaller (64-66).
VMAT
VMAT is the intensity modulated arc form of 3D conformal treatment and their relationship is similar to that described above between IMRT and 3D static fields. Interplay may still play a role in this delivery technique and similar results with normal tissue and dose gradient have been shown, therefore the same considerations for the use of VMAT in the lung should be taken into account (67-69).
Other delivery techniques
Depending on the equipment and device used, other techniques may be available that mimic any one of these four mentioned above. Different delivery machines have different degrees of freedom and ability to adjust for target motion (70). CyberKnife with its possibly fiducial-less tumor tracking and nonisocentric delivery have become a popular method of lung SBRT (71,72). Even TomoTherapy units have been used for lung SBRT in many places as well (73,74). Other devices used are newer and are still being tested clinically by centers who have implemented those machines. It should be noted that recent emphasis has been placed on lung SBRT delivered with very high dose-rate. Due to the availability of linear accelerators without flattening filters, very high dose rates have become available and are being systematically employed in various centers around the world for lung SBRT treatment (75,76).
Treatment delivery
In today’s image guidance age, treatment delivery consists of two parts:
- Localization of target;
- Radiation delivery.
Localization
The treatment delivery process begins with patient immobilization and setup just as it occurred during simulation. The treating therapists spend time to reproduce as closely as possible the setup that was acquired at simulation all the way down to exact vacuum pressure numbers and respiratory fiducial placement. Then, the patient is roughly aligned at the treatment isocenter based on external markings and imaging is performed. The imaging utilized can vary between sites; however, consistency typically exists for sites using similar machines for delivery. For traditional linear accelerators, cone-beam CT (CBCT) is the most common. However, relatively recently 4D CBCT has become available, but has not yet been adopted for widespread use even for SBRT lung. Fluoroscopy-based systems exist for traditional linear accelerators, but are most often utilized with other stereotactic machines. These systems are most useful when attempting to track tumor motion during delivery using implanted fiducials. Vendors are beginning to provide systems where the tumor can not only be tracked during delivery, but the collimating device or treatment couch can actually adjust to the actual tumor position. Currently, this “real-time” tracking requires the use of fiducials. Other systems may use megavoltage CT or even simplified magnetic resonance imaging. The latter is in development with current linear accelerator vendors and would be ideal due to improved soft tissue contrast and zero imaging dose (39,40,43).
Regardless of the imaging technique utilized, imaging must occur prior to treatment and then the patient will be adjusted based on the comparison of the current image to reference images created during simulation and planning. The size of these shifts often dictates whether imaging should be performed again before treatment. In some departments, shifts >5 mm require a repeat CBCT before treatment to verify correct localization. Repeat imaging is also sometimes performed prior to adjusting couch rotation for noncoplanar beams and at the end of treatment. If respiratory gating is to be used, that system must be set up and synchronized with the delivery system before beam on. The same must also occur for any fiducial/tumor tracking systems.
Radiation delivery
Many departments require the presence of the physician and physicist during stereotactic hypofractionated procedures. Once the staff is present and pre-treatment setup and imaging is approved, treatment delivery can commence. It is important that all staff is aware of both the patient and the necessary monitoring systems. Any significant patient motion or system malfunction such as gating may require a pause in treatment and a repeat of setup and imaging. Treatments often take time on the order of 20-90 min from setup to delivery completion depending on staff familiarity, plan complexity or delivery technique, and delivery mechanism. The use of flattening filters in linear accelerators has been shown to significantly affect total delivery time (75,76).
Summary
SBRT to the lung requires great effort on the part of all the radiation oncology staff. Its success and not to mention convenience for the patient cannot be ignored. Each person involved must be sure to invest in the necessary attention to detail and consideration of challenges that SBRT lung requires. Even though its success in lung cancer has been shown, implementation and use of this technique carries with it a significant amount of risk for harm even when the procedure is performed properly (77).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyudmila Bazhenova and Ajay Pal Singh Sandhu) for the series “Recent advances in radiotherapy and targeted therapies for lung cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.08.11). The series “Recent advances in radiotherapy and targeted therapies for lung cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [PubMed]
- Haffty BG, Goldberg NB, Gerstley J, et al. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1988;15:69-73. [PubMed]
- Sibley GS, Jamieson TA, Marks LB, et al. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998;40:149-54. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Krol AD, Aussems P, Noordijk EM, et al. Local irradiation alone for peripheral stage I lung cancer: could we omit the elective regional nodal irradiation? Int J Radiat Oncol Biol Phys 1996;34:297-302. [PubMed]
- Williams TE, Thomas CR Jr, Turrisi AT 3rd. Counterpoint: better radiation treatment of non-small cell lung cancer using new techniques without elective nodal irradiation. Semin Radiat Oncol 2000;10:315-23. [PubMed]
- Robertson JM, Ten Haken RK, Hazuka MB, et al. Dose escalation for non-small cell lung cancer using conformal radiation therapy. Int J Radiat Oncol Biol Phys 1997;37:1079-85. [PubMed]
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2005;61:318-28. [PubMed]
- Slotman BJ, Antonisse IE, Njo KH. Limited field irradiation in early stage (T1-2N0) non-small cell lung cancer. Radiother Oncol 1996;41:41-4. [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946-55. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Hall EJ. Radiobiology for the Radiologist. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2000.
- Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 2003;22:5897-906. [PubMed]
- Henry B, Möller C, Dimanche-Boitrel MT, et al. Targeting the ceramide system in cancer. Cancer Lett 2013;332:286-94. [PubMed]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004;116:205-19. [PubMed]
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155-9. [PubMed]
- Rusthoven KE, Hammerman SF, Kavanagh BD, et al. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol 2009;48:578-83. [PubMed]
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824-30. [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- . Pretreatment evaluation of non-small-cell lung cancer. The American Thoracic Society and The European Respiratory Society. Am J Respir Crit Care Med 1997;156:320-32. [PubMed]
- Dwamena BA, Sonnad SS, Angobaldo JO, et al. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s--meta-analytic comparison of PET and CT. Radiology 1999;213:530-6. [PubMed]
- Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:137S-146S. [PubMed]
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S.
- Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005;79:375-82. [PubMed]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [PubMed]
- Schrevens L, Lorent N, Dooms C, et al. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist 2004;9:633-43. [PubMed]
- Bunyaviroch T, Coleman RE. PET evaluation of lung cancer. J Nucl Med 2006;47:451-69. [PubMed]
- Takamochi K, Yoshida J, Murakami K, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer 2005;47:235-42. [PubMed]
- Schmidt-Hansen M, Baldwin DR, Hasler E, et al. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014;11:CD009519 [PubMed]
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500-7. [PubMed]
- Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg 2003;126:1943-51. [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [PubMed]
- Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. Endoscopic ultrasound: it's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol 2008;14:3028-37. [PubMed]
- Prenzel KL, Mönig SP, Sinning JM, et al. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest 2003;123:463-7. [PubMed]
- Catalano MF, Sivak MV Jr, Rice T, et al. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994;40:442-6. [PubMed]
- Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc 1997;45:474-9. [PubMed]
- Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Surg 2003;238:180-8. [PubMed]
- Rule WG, Jain S, Boike TP. Stereotactic body radiation therapy. In: Khan FM, Gerbi BJ, eds. Treatment Planning in Radiation Oncology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012:278-97.
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [PubMed]
- Khan FM, Gibbons JP. The physics of radiation therapy. 5th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins, 2014:18.
- Solberg TD, Balter JM, Benedict SH, et al. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: Executive summary. Pract Radiat Oncol 2012;2:2-9. [PubMed]
- Bova, F J. Cranial Radiosurgery. Treatment Planning in Radiation Oncology. Ed. FM Khan and BJ Gerbi. 3rd. Philadelphia: Lippincott Williams & Wilkins, 2012:259-77.
- Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006;33:3874-900. [PubMed]
- Fuss M, Salter BJ, Rassiah P, et al. Repositioning accuracy of a commercially available double-vacuum whole body immobilization system for stereotactic body radiation therapy. Technol Cancer Res Treat 2004;3:59-67. [PubMed]
- Blomgren H, Lax I, Göranson H, et al. Radiosurgery for Tumors in the Body: Clinical Experience Using a New Method. Journal of Radiosurgery 1998;1:63-74.
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother Oncol 2000;57:225-36. [PubMed]
- Abdelnour AF, Nehmeh SA, Pan T, et al. Phase and amplitude binning for 4D-CT imaging. Phys Med Biol 2007;52:3515-29. [PubMed]
- Li H, Noel C, Garcia-Ramirez J, et al. Clinical evaluations of an amplitude-based binning algorithm for 4DCT reconstruction in radiation therapy. Med Phys 2012;39:922-32. [PubMed]
- Didierlaurent D, Ribes S, Batatia H, et al. The retrospective binning method improves the consistency of phase binning in respiratory-gated PET/CT. Phys Med Biol 2012;57:7829-41. [PubMed]
- Castillo SJ, Castillo R, Castillo E, et al. Evaluation of 4D CT acquisition methods designed to reduce artifacts. J Appl Clin Med Phys 2015;16:4949. [PubMed]
- Cerviño LI, Chao AK, Sandhu A, et al. The diaphragm as an anatomic surrogate for lung tumor motion. Phys Med Biol 2009;54:3529-41. [PubMed]
- Tian Y, Wang Z, Ge H, et al. Dosimetric comparison of treatment plans based on free breathing, maximum, and average intensity projection CTs for lung cancer SBRT. Med Phys 2012;39:2754-60. [PubMed]
- Ehler ED, Tomé WA. Lung 4D-IMRT treatment planning: an evaluation of three methods applied to four-dimensional data sets. Radiother Oncol 2008;88:319-25. [PubMed]
- Liu J, Wang JZ, Zhao JD, et al. Use of combined maximum and minimum intensity projections to determine internal target volume in 4-dimensional CT scans for hepatic malignancies. Radiat Oncol 2012;7:11. [PubMed]
- Grosu AL, Piert M, Weber WA, et al. Positron emission tomography for radiation treatment planning. Strahlenther Onkol 2005;181:483-99. [PubMed]
- de Figueiredo BH, Antoine M, Trouette R, et al. Use of FDG-PET to guide dose prescription heterogeneity in stereotactic body radiation therapy for lung cancers with volumetric modulated arc therapy: a feasibility study. Radiat Oncol 2014;9:300. [PubMed]
- Rong Y, Bazan JG, Sekhon A, et al. Minimal Inter-Fractional Fiducial Migration during Image-Guided Lung Stereotactic Body Radiotherapy Using SuperLock Nitinol Coil Fiducial Markers. PLoS One 2015;10:e0131945 [PubMed]
- Nuyttens JJ, Prévost JB, Praag J, et al. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: Marker placement and early results. Acta Oncol 2006;45:961-5. [PubMed]
- International Commission on Radiation Units and Measurements. ICRU Report 83 Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT). Journal of the ICRU 10.1, 2010.
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679-94. [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [PubMed]
- Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 2008;18:240-3. [PubMed]
- Emami B. Tolerance of normal tissue to therapeutic radiation. Reports of Radiotherapy and Oncology 2013;1:35-48.
- Ong CL, Verbakel WF, Cuijpers JP, et al. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010;97:437-42. [PubMed]
- Bortfeld T, Jiang SB, Rietzel E. Effects of motion on the total dose distribution. Semin Radiat Oncol 2004;14:41-51. [PubMed]
- Rao M, Wu J, Cao D, et al. Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using intensity modulated radiotherapy and volumetric modulated arc therapy Int J Radiat Oncol Biol Phys 2012;83:e251-6. [corrected]. [PubMed]
- McGrath SD, Matuszak MM, Yan D, et al. Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: A dosimetric and treatment efficiency analysis. Radiother Oncol 2010;95:153-7. [PubMed]
- Brock J, Bedford J, Partridge M, et al. Optimising stereotactic body radiotherapy for non-small cell lung cancer with volumetric intensity-modulated arc therapy--a planning study. Clin Oncol (R Coll Radiol) 2012;24:68-75. [PubMed]
- Ong C, Verbakel WF, Cuijpers JP, et al. Dosimetric impact of interplay effect on RapidArc lung stereotactic treatment delivery. Int J Radiat Oncol Biol Phys 2011;79:305-11. [PubMed]
- Tajima Y, Nakayama H, Itonaga T, et al. Dosimetric evaluation of compensator intensity modulation-based stereotactic body radiotherapy for Stage I non-small-cell lung cancer. Br J Radiol 2015;88:20150122 [PubMed]
- Wang Z, Kong QT, Li J, et al. Clinical outcomes of cyberknife stereotactic radiosurgery for lung metastases. J Thorac Dis 2015;7:407-12. [PubMed]
- Bahig H, Campeau MP, Vu T, et al. Predictive parameters of CyberKnife fiducial-less (XSight Lung) applicability for treatment of early non-small cell lung cancer: a single-center experience. Int J Radiat Oncol Biol Phys 2013;87:583-9. [PubMed]
- Arcangeli S, Agolli L, Portalone L, et al. Patterns of CT lung injury and toxicity after stereotactic radiotherapy delivered with helical tomotherapy in early stage medically inoperable NSCLC. Br J Radiol 2015;88:20140728 [PubMed]
- Casutt A, Bouchaab H, Beigelman-Aubry C, et al. Stereotactic body radiotherapy with helical TomoTherapy for medically inoperable early stage primary and second-primary non-small-cell lung neoplasm: 1-year outcome and toxicity analysis. Br J Radiol 2015;88:20140687 [PubMed]
- Hrbacek J, Lang S, Graydon SN, et al. Dosimetric comparison of flattened and unflattened beams for stereotactic ablative radiotherapy of stage I non-small cell lung cancer. Med Phys 2014;41:031709 [PubMed]
- Li X, Yang Y, Li T, et al. Dosimetric effect of respiratory motion on volumetric-modulated arc therapy-based lung SBRT treatment delivered by TrueBeam machine with flattening filter-free beam. J Appl Clin Med Phys 2013;14:4370. [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327-9. [PubMed]


