
Brachytherapy in lung cancer: a review
Introduction
Metastatic non-small cell lung cancer (NSCLC) remains the leading cause of cancer death in men and the second leading cause of cancer deaths in women worldwide (1). It is a debilitating disease that results in a high burden of symptoms including shortness of breath, hemoptysis, cough and pain resulting in poor quality of life. Intraluminal brachytherapy (ILBT) has been shown to improve patients’ symptoms in some studies. However, its role in the palliation of these patients amidst the other local treatment modalities such as external beam radiotherapy (EBRT), laser and photodynamic therapy (PDT) remains unclear. Effectiveness in palliation and toxicity profile vary widely between cohorts of patients treated with different fractionation schemes making it difficult to compare them. Finally, ILBT has also been used in different ways to treat patients in a radical setting in small series.
We have endeavoured to pursue a systematic review of the literature to evaluate outcomes of patients with lung tumours treated with ILBT alone and/or in combination with other treatment modalities. We have reviewed outcomes of patients treated with diverse fractionation schemes and those treated with a curative intent.
Methods and materials
Literature search
The English and French-language literature from 1980 to June 1st 2015 was reviewed according to PRISMA guidelines (2), using PubMed. All relevant abstracts and articles were thoroughly examined by two independent individuals. Studies were included if they (I) consisted of randomized control trials (RCT), prospective studies or retrospective studies; (II) included patients with biopsy-proven lung cancer and treated with ILBT.
Studies were excluded if they (I) included patients treated for distant lung metastases (II) were non-original studies, i.e., practice guidelines, metaanalyses or systematic review articles (III) did not address patients’ outcomes after treatments of their endoluminal lung cancer; (IV) included patients treated with interstitial brachytherapy; (V) patients were treated post-operatively; (VI) treated patients with low-dose rate brachytherapy (LDR), medium dose rate brachytherapy (MDR) or pulse-dose rate brachytherapy (PDR).
The results were divided into into RCTs, prospective studies and retrospective studies. Although meta-analyses, systematic reviews and practice guidelines were not included in our literature search, these were cross-referenced with our search strategies to ensure a complete set of manuscripts for review. The following keywords and MeSH headings were used: “radiotherapy or irradiation or external beam or radiation” and “palliation or palliative or lung cancer or bronchial or endobronchial or lung malignancy” and “thoracic cancer or lung cancer or bronch- or endobronchial” and “palliation or palliative or lung cancer or bronchial or endobronchial or lung malignancy”.
Results
Randomized control trials describing the role of ILBT alone or in conjunction with other modalities in the palliative management of lung cancer
ILBT allows the delivery of high-dose radiation to the luminal aspect of the tumour and thereby, relieving patients’ symptoms. Few RCTs have attempted to assess the benefit of ILBT in addition to or compared to other treatment modalities, with conflicting results. These are studies with their results are summarized in Table 1.

Full table
Many RCT have randomized patients to EBRT with or without ILBT to evaluate the impact on outcomes with the addition of ILBT.
Huber et al. (8) randomized 93 patients with NSCLC treated with ILBT to a mean delivered dose of 13.4 Gy in 4 weekly fractions or 13.7 Gy in 2 fractions over 3 weeks. The 1-year survival, local control (LC) and fatal hemoptysis rates were not significantly different between the two groups. Huber et al. (7) also randomized 98 patients with inoperable NSCLC to EBRT alone (n=42) compared to EBRT with ILBT (EBRT-ILBT) (n=56). In this trial, although survival rates (and fatal hemoptysis rates) were similar, patients with primary lung squamous cell carcinoma experienced a significantly longer LC when treated with EBRT+ ILBT compared to EBRT alone.
More recently, Langendijk et al. (4) randomized previously untreated NSCLC stage I-IIIB in proximal airways to EBRT to 30 Gy in 10 fractions with or without ILBT to a dose of 15 Gy in 2 fractions delivered weekly. This study showed that the addition of ILBT to EBRT improved the rates of re-expansion of collapsed lung from obstructing tumours in the main bronchus resulting in lower levels of dyspnea. There were improved rates of re-aeration (57% vs. 35%, P=0.001) and mean dyspnea scores (P=0.02) over time in patients treated with EBRT-ILBT compared to those treated with EBRT alone.
Stout et al. (6) randomized 99 patients with inoperable NSCLC to EBRT (30 Gy over 10-12 days) or ILBT (15 Gy). Although patients treated with EBRT had significantly longer survival (9.4 vs. 8.2 months), their dysphagia rates were also higher. ILBT and EBRT both provided similar symptom response rates. Of note, in this study, many patients treated with EBRT also received ILBT and vice versa. Patients’ tumour size response was not reported.
Niemoeller et al. (3) randomized 142 patients with advanced endoluminal centrally located malignant tumours to a different ILBT fractionation schemes, i.e., 15.2 Gy in 4 weekly fractions (n=60) or 14.4 Gy in 2 fractions in 3 weeks. In both groups, survival and symptom response were similar. Interestingly, local tumour response with 2 fractions was significantly higher compared to 4 fractions (median 12 vs. 6 weeks, P<0.015) and fatal hemoptysis rate was lower (12% vs. 18%), although it did not reach statistical significance. Niemoeller et al. attributed the difference in the results to a higher radiation dose per fraction. It is also possible that the larger sample size of Niemoeller’s cohort and the difference in the randomization method—performance status in each group was not described and not accounted for- may explain these different findings.
Chella et al. (5) evaluated the role of ILBT in addition to Nd-YAG laser in a RCT. Their study included 29 patients with NSCLC involving the central airways between Nd-YAG versus Nd-YAG with ILBT. The addition of ILBT to Nd-YAG increased the symptom-free survival (8.5 vs. 2.8 months P<0.05) and decreased the need of further endoscopic treatments (15 vs. 3 further endoscopic treatments, P<0.05). It is possible that these two treatments are in fact complimentary. Indeed, Nd-YAG laser may be used to remove bulky tumours-to relieve symptoms rapidly while delivering ILBT provides a longer symptom-free survival and limit the needs of further interventions.
The current data suggests that in patients with endobronchial disease, ILBT given in addition to EBRT may improve LC and symptoms, especially in patients with collapse lung. When ILBT is delivered without EBRT, the use of Nd-YAG laser may be complimentary because it can remove bulky tumours and relieve symptoms rapidly and while ILBT provides a longer period of symptom-free survival. Further studies are needed to better evaluate and quantify the benefits of the addition of ILBT to EBRT in a palliative setting.
Prospective studies describing the role of ILBT alone or in conjunction with other modalities in the palliative management of lung cancer
Ornadel et al. (9) reported outcomes of 117 patients previously treated patients undergoing with Nd-YAG laser prior to BT if there was significant endobronchial likely to cause lung collapse before ILBT would have time to relieve obstruction or if patients were in acute distress. ILBT dose was of 15 Gy in 1 fraction, prescribed at 1 cm from the source axis. There was an improvement in symptoms in 59% for cough, 50% for dyspnea and 76% for hemoptysis. Of note, patients with prior laser treatments had a statistically significantly higher risk of subsequent fatal hemoptysis.
Muto et al. (10) reported outcomes of 320 patients with stage III NSCLC treated with EBRT (60 Gy in 30 fractions) and with three different schedules of ILBT of 10 Gy in 1 fraction, 14 Gy in 2 fractions and 15 Gy in 3 fractions. The mean OS and rates of symptomatic improvements were not statistically significantly different between the groups. However, the group treated with 3 fractions experienced less toxicity.
Skowronek et al. (11) treated 15 patients with 20-30 Gy of EBRT and a weekly high-dose rate (HDR) brachytherapy (3 fractions of 3.5-10 Gy, at 1 cm from the source). In all patients subjective improvement (regression of all symptoms) was found on the first check-up following treatment. In one case complete remission of the tumour lasted for over 6 months, 9 cases had partial remission. The combination of ILBT and EBRT led to regression of symptoms and improvement of well-being in most patients.
Speiser et al. (12) reported one of the largest series of patients treated with ILBT with or without EBRT. The ILBT dose ranged between 22.5 and 30 Gy in 3 fractions. There was a high symptom response rate ranging between 85% (cough, SOB) and 99% (hemoptysis). The rates of procedure-related complications were low, at 3%.
Finally, Freitag et al. (13) assessed indirectly whether PDT compared to PDT with ILBT improved tumour response. This prospective study included unresectable endobronchial primary bronchogenic carcinoma (n=15) and recurrent lung tumours (n=17). The complete response rate associated with the initial PDT was of 75%. After patient completed ILBT, the CR was of 97%. These results suggest that perhaps delivering ILBT in addition to PDT may improve tumour control. It would be interesting to evaluate how ILBT alone compares to PDT, with the hope of using only one modality of treatment and minimize toxicities.
Most recently, Goldberg et al. (14) reported outcomes of inoperable patients with endobronchial lung cancer treated with ILBT with or without EBRT or chemoradiation. ILBT was delivered with a dose of 14 Gy in 2 fractions. Although the survival was improved in patients with CRT, the mean cough-free survival (4.7 months), mean shortness of breath-free survival (5.8 months) and hemoptysis free-survival (7.7 months) were not statistically significant between groups.
Freitag et al. (13) assessed indirectly whether PDT compared to PDT with ILBT improved tumour response. This prospective study included unresectable endobronchial primary bronchogenic carcinoma (n=15) and recurrent lung tumours (n=17). The complete response rate associated with the initial PDT was of 75%. After patient completed ILBT, the CR was of 97%. These results suggest that perhaps delivering ILBT in addition to PDT may improve tumour control. It would be interesting to evaluate how ILBT alone compares to PDT, with the hope of using only one modality of treatment and minimize toxicities.
All these prospective studies used different fractionation schemes and doses. All series report a good rate of symptom relief with low incidence of toxicities. Further studies are needed to determine the optimal dose fractionation scheme.
Retrospective studies describing the role of ILBT alone or in conjunction with other modalities in the palliative management of lung cancer
Many fractionation schemes have been described in retrospective series. The vast majority of the literature on ILBT discusses its use in the palliation of patients with lung cancers to relieve symptoms such as hemoptysis, cough, dyspnea or atelectasis. The data is summarized in Tables 1,2,3. Its effectiveness in improving symptoms mostly ranges from 43% to 92%, depending on symptoms evaluated. Hemoptysis is the most consistently and effectively palliated symptom, with relief in 70-100% of cases. On the other hand, dyspnea is the symptom the least consistently relieved, with rates ranging from 33-85%.
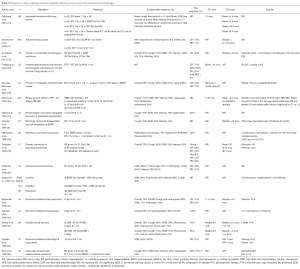
Full table
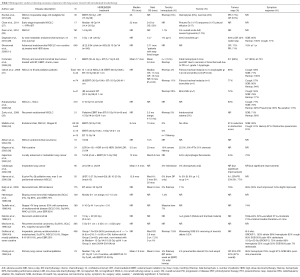
Full table
Technical aspect of ILBT
Prescription point
The prescription point for lung brachytherapy is usually 1 cm from the centre of the source axis. Many authors attempt to treat the entire tumor with the brachytherapy catheters. For tumours in the trachea and mainstem, a prescription point at 1cm from the centre of the source axis is safe. For the segmental bronchi, a prescription point at 0.5 cm from the centre of the source axis is safer. It is not possible to treat the entire tumour with brachytherapy. Dose prescription points beyond what is recommended above can lead to massive cartilage necrosis causing airway- vascular fistula and massive haemoptysis.
Optimal dose fractionation scheme for palliative endobronchial brachytherapy for patients with lung cancer
In a palliative setting, the ideal treatment schedule aims to balance maximal LC and tumour and symptom response with minimal toxicities from ILBT (such as fatal hemoptysis), number of treatments and overall treatment time. There are innumerable fractionation schemes published in the literature with limited studies comparing them to establish superiority of one over the others.
Huber et al. (8) randomized 93 patients with NSCLC to a mean delivered dose of 13.4 Gy in 4 weekly fractions or 13.7 Gy in 2 fractions q3weeks. The 1-year survival, LC and fatal hemoptysis rates were not significantly different between the two groups.
One of the largest study most recent from Niemoeller et al. (3) randomized 142 patients with advanced endoluminal centrally located malignant tumors to a similar fractionation schemes, i.e., 15.2 Gy in 4 weekly fractions (n=60) or 14.4 Gy in 2 fractions over 3 weeks. In both groups, survival and symptom response were similar. Interestingly, local tumour response with 2 fractions was significantly higher compared to 4 fractions (median 12 vs. 6 weeks, P<0.015) and fatal hemoptysis rate was lower (12% vs. 18%), although it did not reach statistical significance. Niemoeller et al. attributed the difference in the results to a higher irradiation dose per fraction. It is also possible that the larger sample size of Niemoeller’s cohort and the difference in the randomization method—performance status in each group was not described and not accounted for- may explain these different findings.
More prospective randomised studies are needed to establish the optimal dose and fractionation can give the best palliation with minimal toxicity in advanced lung cancer.
ILBT-related toxicities
Acute toxicities
One of the most attractive characteristic of ILBT is its a sharp dose fall-off curve. It is thus not surprising that its acute toxicities are relatively limited. Although acute bronchitis or pneumonitis has been reported in up to 46% of patients treated (10,31,43), these episodes were usually self-limited or readily treated with inhaled bronchodilators or steroids. Incidences of rapid necrosis of tumours sometimes requiring bronchoscopic removal of the debris have been reported in up to 5% of cases (26,43); patients generally improved after the procedure. Small risks of procedure-related complications such as pneumothorax, infection/empyema/abscess has been reported in up to 6% of cases (12,19,36,45).
Long-term toxicities
The most significant long-term toxicities include fibrosis causing stenosis, fistulisation and fatal hemoptysis.
Rates of bronchial fibrosis causing stenosis range from 2-56% (31,36,45). In most, patients are asymptomatic or minimally symptomatic thus not requiring any intervention. Fistulisation is a more significant complication, as it may lead to uncontrolled infections that may be fatal, albeit these are rare occurrences (41). Rates of fistula are relatively rare, ranging from 1-11% and were not fatal in most series (10,15,16,23,46).
One of most significant toxicity from ILBT consists of hemoptysis that may be fatal. It can occur as soon as a few weeks after ILBT and as late as almost 1 year post-ILBT. Rates of fatal hemoptysis are highly variable and in some series, can be up to 19-33% (7,21,24,31). Ornadel et al. (9) suggested that prior laser treatments increased the risk of fatal hemoptysis. Dose prescription point is important in preventing fatal hemoptysis although this has not been tested in a clinical setting. Hemoptysis also occurs due to disease progression and invasion of blood vessels by tumour and not necessarily from ILBT. In most series, incidences of massive hemoptysis ranged between 2-10% and were almost invariable fatal.
Role of ILBT alone or in conjunction with other modalities in a radical setting
While the most common use of ILBT remains in a palliative setting, small series have reported outcomes of patients treated with EBBT either alone or as a boost to EBRT alone, as shown in Table 4.
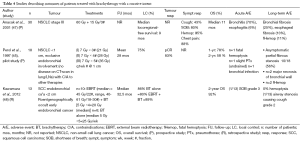
Full table
Kawamura et al. (48) have previously reported outcomes of 13 patients with small endobronchial squamous cell carcinomas treated wither with EBRT and ILBT or ILBT alone. The median ILBT dose was 20 Gy in four fractions and 25 Gy in 5 fractions, respectively. The median dose was 45 Gy (range, 40-61 Gy), delivered at 2 Gy/fraction. The 2-year overall survival (OS) and 2-year LC were 92% and 86%, respectively. The 2-year LC was slightly higher for patients treated with EBRT-ILBT compared to ILBT alone (89% vs. 80%). One patient who experience airway stenosis causing cough (n=1/13) and another patient experience dyspnea grade 3 after treatments.
Perol et al. (45) reported prospective data on outcomes of 18 patients treated with ILBT alone with a dose escalation scheme from 21 to 35 Gy, prescribed at 1cm from the source axis and delivered at 7 Gy per fraction, weekly. The 2-year LC and OS rates were of 75% and 58%, respectively. Moreover, two patients developed major necrosis of their bronchial wall and two patients died after an episode of fatal hemoptysis. When comparing these results to those of patients treated with stereotactic body radiotherapy in other studies (49), patients treated with ILBT had a lower LC and OS rates. Furthermore, the occurrences of fatal toxicities were relatively high. Further studies are needed to evaluate the role of ILBT in a curative setting. Until further evidence is available, ILBT should be used on a case-by-case basis, and offered only when other better-established treatments such as surgery or stereotactic body radiotherapy have been deemed not feasible.
Fernando et al. (50) have reported outcomes of 224 high-risk operable patients with T1-3N0 NSCLC treated with sublobar resection with or without intraoperative brachytherapy (IOBT). The primary endpoint of this study was to assess whether IOBT improved local recurrence rates or not. The dosimetry goal of IOBT was to deliver 100 Gy at 5-7 mm along the central axis of the resection margin. IOBT did not reduce local recurrence rates or time to recurrence (HR 1.01; 95% CI 0.51-1.98, P=0.98) nor did it improve 3-year OS rates (71% vs. 71%, P=0.97)
In summary, the role of ILBT in curative treatments remains investigational. Its use alone to treat radically endobronchial tumours is not well established and should not be routinely practiced other than in a clinical trial or if the patient is unsuited for surgery/radical chemoradiation due to any reason. ILBT may be considered either as a boost for endoluminal tumours or post-operatively, if they are not candidates for EBRT. These treatments should be delivered within a clinical trial to document outcomes of these patients.
Conclusions
In conclusion, the current evidence mainly supports the use of ILBT in a palliative setting mostly in combination with other treatments modality, such as EBRT (most commonly) and Nd-YAG laser. When delivered with EBRT, it improves rates of lung re-oxygenation and LC without significantly increasing toxicities. Its role in a radical setting remains investigational. Further studies are required to determine the optimal dose fractionation scheme.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyudmila Bazhenova and Ajay Pal Singh Sandhu) for the series “Recent advances in radiotherapy and targeted therapies for lung cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.08.08). The series “Recent advances in radiotherapy and targeted therapies for lung cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer W. World cancer report 2014. In: Stewart BW, Wild CP, eds. 2014:630.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [PubMed]
- Niemoeller OM, Pöllinger B, Niyazi M, et al. Mature results of a randomized trial comparing two fractionation schedules of high dose rate endoluminal brachytherapy for the treatment of endobronchial tumors. Radiat Oncol 2013;8:8. [PubMed]
- Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol 2001;58:257-68. [PubMed]
- Chella A, Ambrogi MC, Ribechini A, et al. Combined Nd-YAG laser/HDR brachytherapy versus Nd-YAG laser only in malignant central airway involvement: a prospective randomized study. Lung Cancer 2000;27:169-75. [PubMed]
- Stout R, Barber P, Burt P, et al. Clinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs. external beam radiotherapy in the palliative treatment of inoperable non-small cell lung cancer. Radiother Oncol 2000;56:323-7. [PubMed]
- Huber RM, Fischer R, Hautmann H, et al. Does additional brachytherapy improve the effect of external irradiation? A prospective, randomized study in central lung tumors. Int J Radiat Oncol Biol Phys 1997;38:533-40. [PubMed]
- Huber RM, Fischer R, Hautmann H, et al. Palliative endobronchial brachytherapy for central lung tumors. A prospective, randomized comparison of two fractionation schedules. Chest 1995;107:463-70. [PubMed]
- Ornadel D, Duchesne G, Wall P, et al. Defining the roles of high dose rate endobronchial brachytherapy and laser resection for recurrent bronchial malignancy. Lung Cancer 1997;16:203-13. [PubMed]
- Muto P, Ravo V, Panelli G, et al. High-dose rate brachytherapy of bronchial cancer: treatment optimization using three schemes of therapy. Oncologist 2000;5:209-14. [PubMed]
- Skowronek J, Kubaszewska M, Kanikowski M, et al. HDR endobronchial brachytherapy (HDRBT) in the management of advanced lung cancer--comparison of two different dose schedules. Radiother Oncol 2009;93:436-40. [PubMed]
- Speiser BL, Spratling L. Remote afterloading brachytherapy for the local control of endobronchial carcinoma. Int J Radiat Oncol Biol Phys 1993;25:579-87. [PubMed]
- Freitag L, Ernst A, Thomas M, et al. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax 2004;59:790-3. [PubMed]
- Goldberg M, Timotin E, Farrell T, et al. A prospective analysis of high-dose-rate endobronchial brachytherapy in the palliation of obstructive symptoms in lung cancer patients: A single-institution experience. Brachytherapy 2015; [Epub ahead of print]. [PubMed]
- de Aquino Gorayeb MM, Gregorio MG, et al. High-dose-rate brachytherapy in symptom palliation due to malignant endobronchial obstruction: a quantitative assessment. Brachytherapy 2013;12:471-8. [PubMed]
- Escobar-Sacristán JA, Granda-Orive JI, Gutiérrez Jiménez T, et al. Endobronchial brachytherapy in the treatment of malignant lung tumours. Eur Respir J 2004;24:348-52. [PubMed]
- Ofiara L, Roman T, Schwartzman K, et al. Local determinants of response to endobronchial high-dose rate brachytherapy in bronchogenic carcinoma. Chest 1997;112:946-53. [PubMed]
- Hernandez P, Gursahaney A, Roman T, et al. High dose rate brachytherapy for the local control of endobronchial carcinoma following external irradiation. Thorax 1996;51:354-58. [PubMed]
- Trédaniel J, Hennequin C, Zalcman G, et al. Prolonged survival after high-dose rate endobronchial radiation for malignant airway obstruction. Chest 1994;105:767-72. [PubMed]
- Goldman JM, Bulman AS, Rathmell AJ, et al. Physiological effect of endobronchial radiotherapy in patients with major airway occlusion by carcinoma. Thorax 1993;48:110-4. [PubMed]
- Bedwinek J, Petty A, Bruton C, et al. The use of high dose rate endobronchial brachytherapy to palliate symptomatic endobronchial recurrence of previously irradiated bronchogenic carcinoma. Int J Radiat Oncol Biol Phys 1992;22:23-30. [PubMed]
- Gauwitz M, Ellerbroek N, Komaki R, et al. High dose endobronchial irradiation in recurrent bronchogenic carcinoma. Int J Radiat Oncol Biol Phys 1992;23:397-400. [PubMed]
- Mehta M, Petereit D, Chosy L, et al. Sequential comparison of low dose rate and hyperfractionated high dose rate endobronchial radiation for malignant airway occlusion. Int J Radiat Oncol Biol Phys 1992;23:133-9. [PubMed]
- Sutedja G, Baris G, Schaake-Koning C, et al. High dose rate brachytherapy in patients with local recurrences after radiotherapy of non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1992;24:551-3. [PubMed]
- Burt PA, O’Driscoll BR, Notley HM, et al. Intraluminal irradiation for the palliation of lung cancer with the high dose rate micro-Selectron. Thorax 1990;45:765-8. [PubMed]
- Rochet N, Hauswald H, Stoiber EM, et al. Primary radiotherapy with endobronchial high-dose-rate brachytherapy boost for inoperable lung cancer: long-term results. Tumori 2013;99:183-90. [PubMed]
- Chan MD, Dupuy DE, Mayo-Smith WW, et al. Combined radiofrequency ablation and high-dose rate brachytherapy for early-stage non-small-cell lung cancer. Brachytherapy 2011;10:253-9. [PubMed]
- Zaric B, Perin B, Jovelic A, et al. Clinical risk factors for early complications after high-dose-rate endobronchial brachytherapy in the palliative treatment of lung cancer. Clin Lung Cancer 2010;11:182-6. [PubMed]
- Dagnault A, Ebacher A, Vigneault E, et al. Retrospective study of 81 patients treated with brachytherapy for endobronchial primary tumor or metastasis. Brachytherapy 2010;9:243-7. [PubMed]
- Fortunato M, Feijo S, Almeida T, et al. Endoluminal high dose rate brachytherapy in the treatment of primary and recurrent bronchogenic tree malignancies. Rev Port Pneumol 2009;15:151-64. [PubMed]
- Ozkok S, Karakoyun-Celik O, Goksel T, et al. High dose rate endobronchial brachytherapy in the management of lung cancer: response and toxicity evaluation in 158 patients. Lung Cancer 2008;62:326-33. [PubMed]
- Kubaszewska M, Skowronek J, Chichel A, et al. The use of high dose rate endobronchial brachytherapy to palliate symptomatic recurrence of previously irriadiated lung cancer. Neoplasma 2008;55:239-45. [PubMed]
- Zorlu AF, Selek U, Emri S, et al. Second line palliative endobronchial radiotherapy with HDR Ir 192 in recurrent lung carcinoma. Yonsei Med J 2008;49:620-4. [PubMed]
- Mallick I, Sharma SC, Behera D. Endobronchial brachytherapy for symptom palliation in non-small cell lung cancer--analysis of symptom response, endoscopic improvement and quality of life. Lung Cancer 2007;55:313-8. [PubMed]
- Allison R, Sibata C, Sarma K, et al. High-dose-rate brachytherapy in combination with stenting offers a rapid and statistically significant improvement in quality of life for patients with endobronchial recurrence. Cancer J 2004;10:368-73. [PubMed]
- Magné N, Porsin B, Marcy PY, et al. Reappraisal of the role of endobronchial brachytherapy in the management of lung cancer: ten years’ experience at the centre Antoine-Lacassagne. Cancer Radiother 2003;7:160-5. [PubMed]
- Gejerman G, Mullokandov EA, Bagiella E, et al. Endobronchial brachytherapy and external-beam radiotherapy in patients with endobronchial obstruction and extrabronchial extension. Brachytherapy 2002;1:204-10. [PubMed]
- Kelly JF, Delclos ME, Morice RC, et al. High-dose-rate endobronchial brachytherapy effectively palliates symptoms due to airway tumors: the 10-year M. D. Anderson cancer center experience. Int J Radiat Oncol Biol Phys 2000;48:697-702. [PubMed]
- Hennequin C, Tredaniel J, Chevret S, et al. Predictive factors for late toxicity after endobronchial brachytherapy: a multivariate analysis. Int J Radiat Oncol Biol Phys 1998;42:21-7. [PubMed]
- Taulelle M, Chauvet B, Vincent P, et al. High dose rate endobronchial brachytherapy: results and complications in 189 patients. Eur Respir J 1998;11:162-8. [PubMed]
- Delclos ME, Komaki R, Morice RC, et al. Endobronchial brachytherapy with high-dose-rate remote afterloading for recurrent endobronchial lesions. Radiology 1996;201:279-82. [PubMed]
- Macha HN, Wahlers B, Reichle C, et al. Endobronchial radiation therapy for obstructing malignancies: ten years’ experience with iridium-192 high-dose radiation brachytherapy afterloading technique in 365 patients. Lung 1995;173:271-80. [PubMed]
- Gollins SW, Burt PA, Barber PV, et al. High dose rate intraluminal radiotherapy for carcinoma of the bronchus: outcome of treatment of 406 patients. Radiother Oncol 1994;33:31-40. [PubMed]
- Chang LF, Horvath J, Peyton W, et al. High dose rate afterloading intraluminal brachytherapy in malignant airway obstruction of lung cancer. Int J Radiat Oncol Biol Phys 1994;28:589-96. [PubMed]
- Pérol M, Caliandro R, Pommier P, et al. Curative irradiation of limited endobronchial carcinomas with high-dose rate brachytherapy. Results of a pilot study. Chest 1997;111:1417-23. [PubMed]
- Sutedja T, Baris G, Zoetmulder F, et al. High dose rate brachytherapy improves resectability in squamous cell lung cancer. Chest 1992;102:308-9. [PubMed]
- Anacak Y, Mogulkoc N, Ozkok S, et al. High dose rate endobronchial brachytherapy in combination with external beam radiotherapy for stage III non-small cell lung cancer. Lung Cancer 2001;34:253-9. [PubMed]
- Kawamura H, Ebara T, Katoh H, et al. Long-term results of curative intraluminal high dose rate brachytherapy for endobronchial carcinoma. Radiat Oncol 2012;7:112. [PubMed]
- Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394-9. [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol 2014;32:2456-62. [PubMed]


