Local control rates with five fractions of stereotactic body radiotherapy for primary lung tumors: a single institution experience of 153 consecutive patients
Introduction
Lung cancer remains the number one cause of cancer mortality in both men and women in the United States, despite tremendous improvement in diagnostic as well as therapeutic modalities (1). Only 20% of patients with non-small cell lung cancer (NSCLC) present with early stage or localized disease, although proposed lung cancer screening programs are likely to lead to a relative increase in early stage NSCLC (2). Surgical resection for localized early stage NSCLC remains the standard of care for early stage NSCLC and yields a 5-year survival rate of 60-70% in operable patients. However, surgery is often infeasible or may involve excessive risk for patients with severe co-morbid tobacco-related cardiopulmonary disease or who decline surgery for personal reasons (3). Observation is not typically recommended; as most will die from progressive lung cancer rather than co-morbid diseases (4). Radiotherapy (RT) remains the standard nonsurgical option for early stage lung cancer. However, conventional fractionated radiotherapy (CFRT) delivering 45-66 Gy in 1.8-2.0 Gy fractions has yielded dismal results (5-year survival rate of 10-30%), with the best results seen when local control (LC) is achieved and/or with the delivery of greater doses (5,6).
Early-stage NSCLC is not inherently systemic from diagnosis, but poor LC with conventional daily fractionated RT has led to the development of nonsurgical approaches aimed at increasing survival by improving local tumor ablation (7). Stereotactic body radiotherapy (SBRT) has been developed as a novel modality for early stage NSCLC and has emerged as standard treatment option for medically-inoperable patients. SBRT uses a large number of non-opposing, often non-coplanar beams, with anatomic targeting using a variety of image-guidance radiotherapy (IGRT) modalities to improve target localization (8,9). The potential benefits of SBRT include non-invasive outpatient treatment without the risks associated with surgery, and increased convenience compared to conventional daily RT (10). The initial single institutional, as well as multi-institutional clinical trials, have shown LC rates as high as 98% at 3 years in early stage lung cancer with low incidence of long-term toxicity (3). With SBRT, improved LC rates are achieved which are almost twice as high as would be expected with conventional 6-7 weeks of daily RT. Despite encouraging early results, long-term follow up and evaluation of these patients is required to understand long term control rates and patterns of recurrence, as well as the type, timing, and severity of late toxicities. SBRT offers promising progression free survival rates without significant increased toxicity compared with standard techniques (3,7-12).
While SBRT seems as efficacious as surgical resection (3,13-16), sufficient outcome data comparing these two modalities are lacking. Three phase III studies comparing SBRT vs. surgery in patients with early stage NSCLC were prematurely closed due to slow accrual: the MDACC (stereotactic RT vs. surgery) STARS trial [NCT00840749], the Dutch Radiosurgery or Surgery for Early stage Lung cancer (ROSEL) trial [NCT00687986], and the American College of Surgeons cooperative group trial [NCT01336894]. A recently published pooled analysis of 58 patients from the STARS and ROSEL studies suggested possibly improved 1-year and 3-year overall survival (OS) in SBRT vs. surgery arms, but no significant difference in frequency of local, regional, or distant metastases or recurrence-free survival between the treatment groups (13). As discussed above, SBRT has clearly resulted in superior outcomes vs. conventionally fractionated RT, but whether this would be true with modern staging and treatment approaches is unknown. Findings from population-based studies and propensity matched analysis comparing outcomes of SBRT vs. surgery have shown similar OS and disease specific survival (14,15). The Scandinavian “Stereotactic Precision and Conventional Radiotherapy Evaluation” (SPACE) study which randomized ~102 patients of SBRT (66 Gy in 3 fractions) to conventional RT (70 Gy in 35 fractions) recently closed to accrual (NCT01920789). The Trans-Tasman “Hypofractionated Radiotherapy (Stereotactic) vs. Conventional Radiotherapy for Inoperable Early Stage I Non-small Cell Lung Cancer” (CHISEL) is enrolling patients in a phase III study of SBRT (54 Gy in 3 fractions) vs. conventional radiation therapy (60-66 Gy in 30-33 fractions) (NCT01014130).
The current retrospective study was undertaken to evaluate our institutional results for high-dose SBRT for early stage NSCLC since we began using a five fraction treatment regimen. We sought to better characterize tumor control with a prescribed dose of 50-60 Gy and determine if outcomes from our single institution with a large cohort of patients were comparable to those of published SBRT data.
Patients and methods
Between January 2008 and December 2012, 153 consecutive patients diagnosed with NSCLC were treated with image-guided SBRT. The study was approved by the University of Rochester Medical Center Research Subjects Review Board.
Patient population
Eligibility criteria included patients with newly diagnosed NSCLC, age >18, Karnofsky performance status >70, CT-defined tumor diameter <5 cm, and no other active metastatic sites outside the lungs. All patients were deemed ineligible for surgical resection, or had refused surgery for personal reasons. The work-up included pulmonary function test, contrast enhanced CT of the chest and abdomen and/or FDG-PET/CT, as well as tissue confirmation in the majority of patients. Patients were followed with CT or PET-CT every 3-6 months for post-treatment surveillance. Patients found to have metachronous NSCLC on surveillance imaging were allowed to undergo additional SBRT treatments.
SBRT technique
The SBRT techniques described in detail in previous publications from our group are briefly summarized here (17,18). All patients undergoing initial CT simulation required immobilization with a vacuum cushion device. All patients were treated with the Novalis ExacTrac system (Brain Lab Inc.). The ExacTrac patient positioning platform using infrared reflecting body fiducial markers monitored by two ceiling mounted infrared cameras was used for patient positioning and real-time monitoring. Respiratory motion was minimized by using relaxed expiratory breath hold techniques (in most patients) or shallow breathing (in patients with poor lung function). Patients also underwent a verification CT in the set-up position, which was fused to the planning CT, prior to treatment and after the second fraction to ensure three-dimensional set-up accuracy. The gross tumor volume (GTV) was delineated using CT and fused PET imaging in the majority of cases. The use of arcs and non-coplanar beams was encouraged. Dose volume histograms (DVH) were calculated for the lung (defined as total lung minus GTV), heart, esophagus, spinal cord, and liver. The planning target volume (PTV) was generated using a 7 mm circumferential and 11 mm superior-inferior expansion of the GTV (with no expansion for CTV). The 80% isodose line encompassed the PTV, with isocenter dose defined as 100% of the prescribed dose. The prescribed target dose was determined based on the DVH of normal (uninvolved) lung and surrounding organs. The median prescription dose was 50 Gy in five fractions (range, 40-60 Gy) to isocenter with 80-100% isodose covering 99-100% of PTV. Generally, 95% of the PTV was covered by the 85-95% isodose line. Patients were required to have 1,000 mL of tumor free lung, with a volume of lung receiving >20 Gy (V20) less than 12%. The spinal cord maximum was required to be <4.5 Gy/fraction. Care was taken so that hot spots (i.e., >95% isodose) occurred solely within the GTV. The dose for smaller peripheral tumors was mostly 50-60 Gy and the dose for larger central tumors was mostly 40-50 Gy.
Outcomes/statistics
The primary end point was tumor LC and secondary end points included regional control as well as OS. Actuarial tumor control and survival were calculated using Kaplan-Meier actuarial survival analyses. OS was defined from date of completion of SBRT until death or last follow-up. Patient LC was scored as an event if any treated lesion grew by ≥20% based on the Response Evaluation Criteria In Solid Tumors (RECIST), or a local recurrence (LR) was pathologically confirmed. LC was analyzed per patient, meaning that if a patient had more than one lesion treated, progression of any of the treated lesions was considered a LR. LC was analyzed by tumor size among patients with more than one lesion, treated tumor size represents the largest lesion treated. Among patients who underwent repeat courses of SBRT for new lesions(s), only the LC of the index lesion(s) was considered in this study. Stata version 9.2 was used for all data analysis.
Results
Patient characteristics
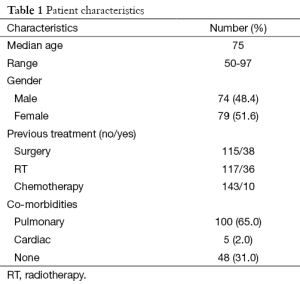
There were 74 males and 79 females. The median age was 75 years (range, 50-97 years). Thirty-eight patients had previous thoracic surgery, 36 had previous thoracic RT and 10 had received systemic chemotherapy in the past (Table 1). Cardiopulmonary co-morbidity was the most common factor for medical inoperability in patients with otherwise technically resectable tumors.
Full table
Tumor characteristics
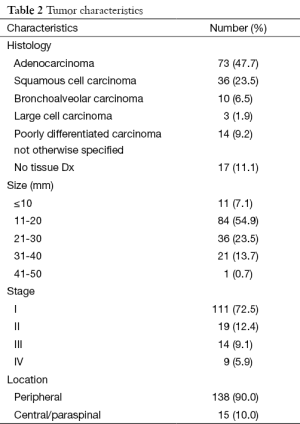
The majority of patients (n=116) underwent bronchoscopic or CT-guided biopsy for tissue diagnosis; however, 17 patients (11%) were considered to be poor risk candidates or refused biopsy for personal reasons. Among the 116 biopsy proven NSCLC, tumor histologies included adenocarcinoma (n=73, 54%), squamous cell carcinoma (n=36, 26%), bronchoalveolar carcinoma (n=10, 7%), large cell carcinoma (n=3, 1.9%), and poorly differentiated carcinoma not otherwise specified (n=14, 10%) (Table 2).
Full table
Tumor size (the largest dimension of the largest target if more than one lesion was treated) was distributed as follows: <10 mm (n=11, 7%); 11-20 mm (n=84, 54%); 21-30 mm (n=36, 23%); 31-40 mm (n=21, 14%); >41 mm (n=1) (Table 2). A total of 72% (n=111) of patients had stage I disease, 12% (n=19) had stage II disease, 9% (n=14) had stage III disease, and 6% (n=9) had stage IV disease (Table 2). Peripherally located tumors accounted for 90% (n=138) of patients vs. 10% (n=15) which were central or paraspinal in location.
Local tumor response
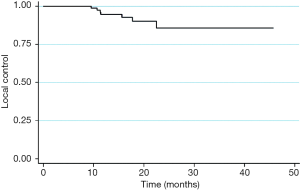
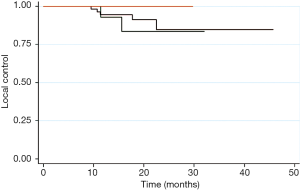
The 1- and 2-year LC rates for all patients were 92% and 85% respectively (Figure 1). For 111 patients with stage I NSCLC, 1- and 2-year LC was 95% and 85%, with all LR occurring within 2 years. LC at 1- and 2-year was 87% for both stage II (n=19) and stage III (n=14) patients, with all LR occurring within 10 months (Figure 2). The 1-year and 2-year LC for oligometastatic stage IV (n=9) patients were 71% each, with all LR occurring within 5 months. The 2-year LC rate among patients with tumors <1 cm was 100% compared to 84% for those with tumor size >1 cm. Tumor histology, prescribed dose, patient age, and prior RT or surgery had no significant impact on LC rates. Prior chemotherapy had a significant negative impact on LC with 1- and 2-year LC of 59% compared to 1- and 2-year LC of 93% and 85%, respectively (P=0.015). However, on multivariate analysis, NSCLC stage was the single most significant factor for LC (P=0.048).
Recurrence
Among the 111 stage I NSCLC patients there were six cases of LR, of which five also developed distant recurrence, as well as an additional eight cases of distant recurrence without LR. Of these 111 patients, five died from NSCLC and two died from causes other than NSCLC.
Toxicity disease
All patients tolerated the SBRT very well. Thirteen patients needed to be treated with steroid inhalers and oral steroids for a short duration. No patient died from treatment-related toxicity.
Discussion
Lung cancer remains one of the most lethal cancers in both men and women in the United States, and accounts for 30% of all cancer deaths (1). Only 20-25% patients with NSCLC patients present with early stage or are deemed to have localized disease. Surgery still remains the standard of care with a 5-year survival rate of 65% seen in stage I patients, along with a 5-year LC rate of 78% (16,19-21).
There are no large published randomized studies comparing SBRT and surgery for operable patients for early stage disease (13) and three phases III randomized studies that were initiated to compare SBRT with surgery in patients with early stage NSCLC were closed early due to slow accrual. These include (I) the STAR trial [NCT00840749], looking at SBRT with Cyber Knife delivering a dose of receive 60 Gy in three fractions to peripheral tumors, and 60 Gy in four fractions to central tumors vs. surgery for stage IA or IB patients (maximum diameter <4 cm); (II) the ROSEL trial [NCT00687986], a Dutch multi-center randomized study of gantry-based SBRT vs. surgery for peripheral stage IA NSCLC; and (III) the ACOSOG trial [NCT01336894]. Although a recently published study with only 58 patients treated either with SABR (n=31) or lobectomy (n=27) showed results in favor of SBRT over surgery (3 years OS and RFS of 95% and 86% for SABR and 79% and 80% for surgery respectively) (13), we are still waiting for the mature data, and in the meantime surgery remains the standard of care (21). The pooled analysis of the STAR and ROSEL trials showed promising estimated OS at 1- and 3-year of 100% and 95% in the SABR group and 86% and 79% in the surgical group (P=0.037), but did not show any significant difference in frequency of local, regional, or distant failure as at 3 years, 96% of patients in the SABR group were free from LR compared with 100% patients with patients in the surgery group (P=0.44) (13).
Initial published phase I and phase II studies from Indiana University showed promising results using SBRT in early stage NSCLC (22-24). In a subsequent update, they reported Kaplan-Meier LC of 88.1% at 3 years, median survival (MS) of 32.4 months, and 3-year OS of 42.7% [95% confidence interval (CI), 31.1-54.3%] at a median follow-up of 50.2 months. For T1 and T2 tumors MS was 38.7 and 24.5 months, respectively, with cancer-specific survival (CSS) at 3 years being 81.7% (25). Baumann et al. reported a 3-year LC rate of 92%, with OS of 86%, 65%, 60%, and CSS of 93%, 88%, and 88% at 1, 2, and 3 years respectively (11).
Review of our institutional experience with five fraction SBRT shows that the 1-, 2-, and 5-year LC rates were 98%, 90%, and 88% respectively; and specifically for 106 patients with stage I NSCLC, 1- and 2-year LC was 95% and 85%. Traditionally, we have been prescribing the SBRT dose to the isocenter with a median prescription dose of 50 Gy in five fractions (range, 40-60 Gy) with 80-100% isodose covering 95% of PTV. Our study shows excellent control rates comparable to other studies, although the total dose in our study is less than other authors who prescribe dose to a volume or to the isocenter (3,26,27). In addition to the excellent control rates with lower total doses of SBRT, our patients did not experience any significant acute or late grade III/IV radiation toxicity.
Onishi et al. published a large retrospective review of 257 stage I resectable patients from 14 centers in Japan showing 5-year actuarial LC rates of 84% for patients treated with SBRT receiving a BED of 100 Gy or more (based on assumed tumor a/b of 10), and 37% for those receiving less than 100 Gy. This dose-response relationship corroborates with that seen with conventionally fractionated radiation. There was no difference in the LR rates of squamous cell carcinoma and adenocarcinoma with a 71% 5-year OS for medically operable patients receiving the higher dose range with relatively low rates of radiation toxicity (3,26). In our current study, the BED doses ranged from 72-100 Gy for central tumors (n=15, 10%) and 96-132 Gy BED (n=138, 90%) for peripheral tumors with no statistically significant differences in LC rates. One possible explanation could be the limited number of patients with central tumors.
In order to determine predictors of LC and OS, many authors have looked at tumor location in the chest, T stage, GTV, histology, laterality, pulmonary function tests, sex, age, cardiac vs. pulmonary cause of inoperability, oxygen dependence, performance status at treatment, ongoing smoking, and PTV. There was no factor significantly predicting OS in the univariate analysis, although some authors pointed out that T size was important (24); however, a subsequent study from their center showed that the tumor size did not have significant impact on survival (P=0.712) (25). Tumor histology, prescribed dose, patient age, and prior RT or surgery had no significant impact on LC rates. However, progression of disease affected OS and CSS negatively (P<0004, and P<0.00001 respectively).
In a series by Fakiris and colleagues from Indiana University, the regional (nodal) and distant recurrence occurred in 6 (8.6%) and 9 patients (12.9%), respectively (25). Onishi et al. reported that LR, lymph node metastases, and distant metastases occurred in 8 (9.2%), 13 (14.9%), and 19 cases (21.8%), respectively (27). Among our stage I NSCLC patients, 6 of 111 developed LF, and 13 developed distant failure (of whom 5 also developed LF). Of these 111 patients, 5 died from NSCLC and 2 died from causes other than NSCLC. Nath et al. reported nodal failures in 3 of 46 evaluable patients (7%) with actuarial 24-month nodal control being 91% (95% CI, 81-100%), and the cumulative incidence of nodal failure being 6% at 24 months. Factors thought to be potentially associated with nodal failure showed no variables associated with nodal control including use of PET imaging (P=0.61), dose per fraction (P=0.89), lesion position (P=0.89), histology (P=0.72), and lesion size (P=0.16) (9).
There was no significant survival difference between patients with peripheral vs. central tumors (MS 33.2 vs. 24.4 months, P=0.697). Grade 3 to 5 toxicity occurred in 5 of 48 patients with peripheral lung tumors (10.4%) and in 6 of 22 patients (27.3%) with central tumors (25). Chang et al. treated a series of 27 centrally or superiorly located lesions with a slightly more modest dose of 40-50 Gy in four fractions. At a median of 17 months, there was no LR seen in the 20 patients receiving 50 Gy (BED 112.5 Gy). There were three cases of grade 2-3 skin/chest wall toxicity and one brachial plexopathy related to a large volume of plexus receiving 40 Gy. However, there was no observed grade 3 pulmonary or esophageal toxicity (28). Our patients tolerated SBRT very well. Thirteen of our patients needed to be treated with steroid inhalers/oral steroids for a short duration. There was no grade III-V toxicity. One patient was noted to have a rib fracture that was treated with analgesics alone.
We used SBRT to treat stage II or III patients, as well some metachronous/oligometastatic lesions, not amenable to surgery or chemotherapy (7). Our studies showed an excellent LC rate of 87% for both stage II and III at 1 and 2 years. The LFs were seen around 10 months in these groups. The 1- and 2-year LC for stage IV were 71% each. Treatment of locally advanced or even metastatic NSCLC with SBRT combined with medical therapies is an area of interest with several institutional studies investigating its use as primary or oligometastatic tumor control in combination with adjuvant chemotherapy [NCT01899989] or even concurrent targeted molecular agents (29). Memorial Sloan-Kettering Cancer Center is currently enrolling patients on a phase I dose escalation study to determine the maximum tolerated dose of SBRT to gross tumor followed by chemotherapy for stage IIA-IIIA NSCLC [NCT01711697].
The RTOG has performed several non-randomized clinical trials investigating the safety and efficacy of SBRT in both inoperable and operable patients. RTOG 0236 was a phase II trial enrolling medically inoperable patients with early stage NSCLC outside the zone of the proximal tracheobronchial tree treated with SBRT to a dose of 60 Gy in three fractions without heterogeneity corrections. Outcomes were excellent with a remarkable 97.6% primary tumor control at median follow up of 34.4 months among 55 evaluable patients (30). A recent 5-year update confirmed excellent primary control of 93% as well as involved lobar control of 80%; however, regional and distant failure remained significant issues with 26% DFS and 40% OS (31). More recently, RTOG 0618 enrolled medically operable patients with similar early stage NSCLC tumors treated with the same SBRT technique yielding an excellent primary tumor control of 92.7%; however, involved lobar control was unexpectedly low at 80.8% at 2 years (32). As the preceding RTOG trials excluded tumors located within the proximal tracheobronchial tree due to concern for risk for severe toxicity, RTOG 0813 was implemented as a dose escalation study to determine the maximum tolerated dose of SBRT when treating tumors within the proximal tracheobronchial tree or adjacent to mediastinal or pericardial pleura. The starting fractional dose was 10 Gy with an increase in 0.5 Gy increments up to 12 Gy over a total course of five fractions [NCT00750269].
In general, limitations of our study include being retrospective in nature as well as marked variation in terms of tumor primary site, size, and histology. Because the majority (90%) of patients were treated with the same dose (60 Gy in five fractions), and the dose range was not large due to smaller number in the 40-50 Gy in five fractions group, we could not adequately analyze a dose-response relationship. Nevertheless, we are able to report promising LC and survival outcomes in this cohort of patients treated with NSCLC with five fractions of SBRT.
Conclusions
SBRT using Novalis/4D techniques for primary lung cancer seems to be very safe and well tolerated, with no grade III/IV toxicity in our study. It offers excellent LC in medically-inoperable NSCLC patients, with treatment during the early stage of the disease determined as the most significant predictor of LC on multivariate analysis.
Acknowledgments
We thank Mrs. Laura Finger for editorial assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyudmila Bazhenova and Ajay Pal Singh Sandhu) for the series “Recent advances in radiotherapy and targeted therapies for lung cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.08.01). The series “Recent advances in radiotherapy and targeted therapies for lung cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Rochester Medical Center Research Subjects Review Board. Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. What are the key statistics about lung cancer? Available online: http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics
- Henschke CI, Yip R, Yankelevitz DF, et al. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158:246-52. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [PubMed]
- McGarry RC, Song G, des Rosiers P, et al. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest 2002;121:1155-8. [PubMed]
- Sibley GS, Jamieson TA, Marks LB, et al. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998;40:149-54. [PubMed]
- Dosoretz DE, Galmarini D, Rubenstein JH, et al. Local control in medically inoperable lung cancer: an analysis of its importance in outcome and factors determining the probability of tumor eradication. Int J Radiat Oncol Biol Phys 1993;27:507-16. [PubMed]
- Salazar OM, Sandhu TS, Lattin PB, et al. Once-weekly, high-dose stereotactic body radiotherapy for lung cancer: 6-year analysis of 60 early-stage, 42 locally advanced, and 7 metastatic lung cancers. Int J Radiat Oncol Biol Phys 2008;72:707-15. [PubMed]
- Martin A, Gaya A. Stereotactic body radiotherapy: a review. Clin Oncol (R Coll Radiol) 2010;22:157-72. [PubMed]
- Nath SK, Sandhu AP, Kim D, et al. Locoregional and distant failure following image-guided stereotactic body radiation for early-stage primary lung cancer. Radiother Oncol 2011;99:12-7. [PubMed]
- Walsh J. Stereotactic Body Therapy Radiation for the Treatment of Early Stage Non Small Cell Lung Cancer. Available online: http://www.medscape.com/viewarticle/748968
- Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol 2006;45:787-95. [PubMed]
- Beitler JJ, Badine EA, El-Sayah D, et al. Stereotactic body radiation therapy for nonmetastatic lung cancer: an analysis of 75 patients treated over 5 years. Int J Radiat Oncol Biol Phys 2006;65:100-6. [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [PubMed]
- Okunieff P, Petersen AL, Philip A, et al. Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol 2006;45:808-17. [PubMed]
- Singh D, Chen Y, Hare MZ, et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis 2014;6:369-74. [PubMed]
- Nesbitt JC, Putnam JB Jr, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg 1995;60:466-72. [PubMed]
- Kelsey CR, Boyd JA, Hubbs JL, et al. Local/regional recurrence following surgery for early-stage lung cancer: a 10-year experience with 975 patients. J Clin Oncol 2008;26:7542.
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236-71. [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946-55. [PubMed]
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010-5. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [PubMed]
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824-30. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. RTOG 0236: Stereotactic body radiation therapy (SBRT) to treat medically inoperable early stage lung cancer patients: American Society for Therapeutic Radiology and Oncology (ASTRO) Annual Meeting, Chicago, IL. Int J Radiat Oncol Biol Phys 2009;75:S3.
- Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol 2013;31:abstr 7523.