RADIANCE—A planning software for intra-operative radiation therapy
Introduction
Intra-operative radiation therapy (IORT) allows physicians to deliver a concentrated dose of radiation to the tumor site immediately after removal, helping to eradicate the microscopic residual tumor cells or, in case removal is not possible, allows reduction of the size of the tumor. A clear delimitation of the area to treat, the possibility of moving temporarily normal tissues to spare them from radiation, the reduced interaction with skin or other organs and a greater anti-tumoral efficacy make IORT a very effective technique (1,2).
IORT has been in clinical use since the 70s with increasing interest in the last years as shown in the last published statistics (3,4). The availability of mobile linear accelerators (LINACs) and miniature low-energy X-ray machines which avoid patient transportation from the Operating Room (OR) to the bunker and the availability of recent large pooled analysis and randomized trials has boosted the adoption of the technique. In spite of this penetration, the number of IORT procedures is quite low with respect to other radiotherapy procedures.
From the technical perspective, the reasons why this is happening are manifold:
- It is a procedure that involves a great deal of organization in terms of structural and human resources: OR and accelerator synchronized use, smooth communication and collaboration of all the intervening actors such as surgeons, anesthetists, oncologists, radio-physicists, nurses, etc.; in cases where a mobile accelerator is not available the patient needs to be transported to the accelerator’s room. No information technology infrastructure is supporting the procedure, unlike external radiation therapy, with the subsequent implication of having the treatment device isolated from the radiation therapy department infrastructure.
- The lack of a treatment planning system (TPS), considered a necessary step in external radiotherapy, means that the effects of beam misalignment, gaps, bolus, changes in penumbra, and tissue inhomogeneities in realistic patient geometries are not properly investigated (5). Dose planning is not optimized to patient specific tumor and surrounding tissue as requested by Euratom Directive 97/43 (6).
- Lack of standardization. Large randomized trials for intraoperative radiotherapy in breast [ELIOT (7) and TARGIT (8,9)] have produced some guidelines in IORT (such as prescription dose and depth). Medical physics professional associations have also provided guidelines such as the Italian initiative (10) or the reports from the AAPM (5,11). In spite of that, technical aspects have not been adopted globally yet. For instance, the majority of times [with fewer exceptions like (12)], the tumor bed volume is not accurately delineated before treatment or it is estimated in the OR right before radiation, or tissue heterogeneity is not considered. In the end, the institutions involved did not follow a consensus guideline to perform IORT and the procedure was considered at some point very artisanal. A simulation software which would be able to plan and record the procedure will definitely enhance the reproducibility into IORT.
- Lack of documentation. Registration of all parameters involved in the procedure and of the administered dose are most of times not recorded within the Hospital Information System: more specifically detailed information on the anatomy receiving the radiation, DVH and maximum and minimum dose in the PTV as requested by ICRU 72 (13) are only available in handwritten reports. Or, if they are recorded, not in a standard way (14), which could be utilized in conjunction with complementary information coming from other treatments (such as external radiotherapy).
The aim of our research activities is to address the technology gap between IORT and other surgical/radiotherapeutical techniques on the following areas:
- Dosimetric algorithms for IORT which deal with heterogeneity corrections;
- Intraoperative images;
- Surgical Navigation systems;
- Automatic segmentation;
- Fusion and registration of medical images;
- Surgical simulation;
- Dose summation;
- Interoperability standards (DICOM.RT).
The first proof-of-concept was developed by the research group at Hospital Gregorio Marañón. Their initial prototype for a TPS for IORT (15) (Figure 1) did not consider heterogeneity corrections or 3D dose volumes computation (only 2D cross-sections). Moreover, at that time, there was no intention of turning this prototype into a certified medical device.
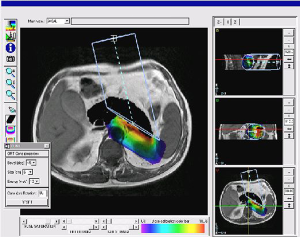
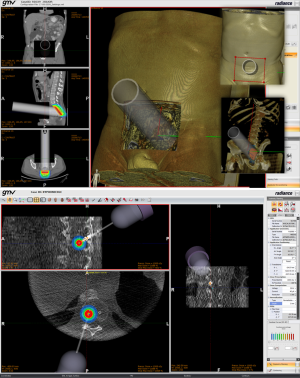
In 2007, GMV and Hospital Gregorio Marañón began a project to fully redesign the first prototype and developed the first release of a TPS for electron based IORT (IOERT) called RADIANCE. The underlying idea was that simulating the IOERT procedure was feasible by displaying the virtual position of the applicator superimposed on the patient’s computed tomography (CT) or magnetic resonance image. With this approach, the treatment parameters could be predefined depending on the patient’s anatomy, and the radiation oncologist (RO) could improve the preoperative planning for the procedure. This tool used experimental data acquired in a water phantom. Measurements were obtained for every combination of applicator diameter-bevel-beam energy, and a 3D dose volume was generated with these measurements. The 3D dose volume was later superimposed on a pre-operative image of the patient (Figure 2) and information could be obtained about the coverage of the dose. However, although water measurements were a good starting point towards providing a planning tool to IORT, they did not take into account the behavior of electrons in tissues with densities different than water.
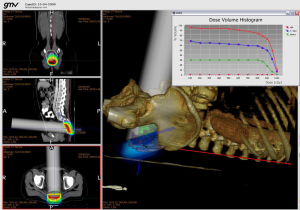
In 2009, we enlarged the research team working in RADIANCE involving oncologists, physicists and engineers, building a research consortium composed of private companies (GMV, Técnicas Radiofísicas), medical institutions (Hospital Universitario Gregorio Marañón, Consorcio Hospitalario Provincial de Castellón, Clínica La Luz, Hospital Ramón y Cajal) and universities (Universidad Politécnica de Madrid, Universidad Complutense de Madrid, Universidad Rey Juan Carlos, Universidad de Valencia, Universidad de Granada), which have worked together in making what RADIANCE is today (Figure 3).
Material and methods
RADIANCE allows the user to simulate the IORT treatment by loading and visualizing CT images of a patient and finding the best parameters involved (applicator’s geometry, accelerator’s parameters, etc.) so that the dose deposit is maximized on the tumor or tumor bed while being minimized on the regions to protect.
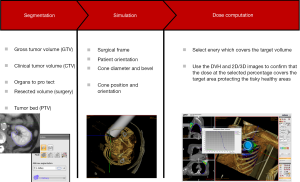
Each of the steps involved during planning with radiance is summarized in the following way (Figure 4):
- Beam modeling and CT calibration;
- Loading of 3D medical images in DICOM format;
- Navigation on the patient to determine course of action;
- Identification of regions of interest, including Gross Tumor Volume (GTV), Clinical Target Volume (CTV), organs at risk;
- Determination of Surgical Frame, to determine the access to the patient;
- Definition of the resection Surgical Target Volume (STV);
- Definition of the Planning Target Volume (PTV) removing the STV from the CTV;
- Simulation bolus, protections, air, etc. ;
- Optimization of IORT parameters with the help of the Dose Volume Histogram (DVH);
- Reporting.
Beam modeling and CT calibration
The user needs to model the accelerator used for treatment as well as determining the correct mapping from Hounsfield Units found in CT images to material densities. RADIANCE has a Beam and CT calibration modeling tool that takes care of that.
The user needs to input the type of LINAC to be used. RADIANCE already contains a template for all possible machines which will make the introduction of data (measurements) used for modeling easier and quicker. The user needs to indicate all the possible applicators to be used and provide a minimum set of measurements as requested by the tool, the extra information that the user is able to provide will also be used and will make the algorithms to have a better accuracy. After all this data is entered, RADIANCE will start to model the accelerator, once this process is finished, the user will be given statistics about the accuracy of the modeling. The user can improve the introduced data (by modifying the current or adding more) in order to achieve an improved accuracy.
The CT calibration model is calculated based on input data obtained from scanning a phantom and determining the mapping of material density to Hounsfield Units. This mapping will be later applied by the dose computation algorithms.
Load images
RADIANCE works mainly with DICOM images, which can be transferred from a PACS system or loaded from the local drive. Although RADIANCE works with images of any image modality, heterogeneous versions of the dose computations algorithms will only be available for CT images as they depict density of tissues. For the rest of modalities, RADIANCE assumes that the whole medium is water.
MPR & Volume rendering; measurements
Like in any radiological workstation, RADIANCE shows by default the three orthogonal cross-sections as well as a 3D view of the patient. The user can navigate through the cross-sections in order to inspect the location of the lesions or the area to irradiate. The 3D volume rendering view provides complementary information as the user can determine the location/orientation of the applicator with respect to the patient in a three-dimensional manner. Enhancement of these views is possible by modifying the windowing contrast parameters for cross-sections or by changing the opacity thresholds for the 3D rendering view.
RADIANCE also provides measurement tools for distances (useful to determine the distance from the surface of the applicator to the tumor bed, allowing choosing the best parameters of the dose simulation to cover the region of interest), or angles (useful to select the required bevel applicator in IOERT).
Contouring
Manual and semiautomatic tools are provided. The user may only need to contour a few cross-sections of the region of interest and RADIANCE can perform a 3D interpolation of these contours. The user may modify, improve or delete any contour at any point during the simulation. RADIANCE allows loading preexisting contours in DICOM RT struct files.
RADIANCE allows assigning a specific density value to a contoured region, this implies that the dose computation algorithms will consider the information within that region to be a tissue with that density, therefore overriding the original density found in the image.
Another interesting feature is the ability to resect contoured regions. Resected regions are considered regions removed from the original image and filled with air, therefore the density of air is assigned. This is particularly interesting when simulating cases where it is likely there will be an interface of air between the applicator and the tumor bed (i.e., near cavities where the applicator cannot be directly in contact with the tumor).
Simulation
RADIANCE allows the user to select and modify the parameters that optimize treatment. Based on the modeled accelerator, the user can inspect all the available applicators and choose the one that he/she considers appropriate. This applicator needs to be positioned in an optimum place, to do that, the user can input the location and orientation of the applicator in the control panel of RADIANCE and/or by using the mouse. The applicator is visible all time in all cross-sections and in the 3D view.
Positioning of the applicator takes into account the degrees of freedom of the modelled LINAC. So, for conventional LINACs, the orientation of the applicator is constrained for the angles of the gantry, collimator and bed selected by the user. In mobile LINACs there is no such restriction on the orientation of the applicator.
The user may add a “surgical frame”, which is way to simulate the incision area as visualized by the surgeon. This area is automatically considered a resected area (from RADIANCE perspective) and it will therefore be filled with air. This is a fine example of communication between the professionals involved in IORT and helps the oncologists to do the planning with feedback from the surgeon about the feasible positioning of the applicator.
Once the applicator is positioned and the dose is calculated, isodose curves can be visualized in all cross-sections as curves or filled curves and as surfaces in the 3D rendering view. The user can specify the number and values of the isodose curves to visualize and can also use an interactive tool to select the curves whose values are higher than a threshold.
Dose calculation algorithms
Three algorithms, as it will be described in the next section, are implemented in RADIANCE: Pencil Beam (PB) and Monte Carlo (MC) for IOERT and Hybrid Monte Carlo (Hybrid MC) for low energy photons. Since RADIANCE is able to work on any image modality, for those different than CT, these algorithms consider that all image information is water. PB runs in times up to 4 seconds, while MC and Hybrid MC in the order of a few minutes.
MC and Hybrid MC parameters can be adjusted by the user to determine a tolerance error value or the number of simulated particles, respectively.
Dose prescription
The user can input the prescribed dose and the isodosis of reference. These parameters can be modified and changes will take effect in the DVH and the display of isodosis.
DVH calculation
A DVH is calculated after dose calculation to provide some means to determine the level of optimization of the current plan. The DVH is recalculated any time the normalization parameters change. Within the DVH, RADIANCE shows the maximum and minimum dose in the PTV as requested by ICRU 72 (13).
Report
All generated information during the simulation can be stored in the report. The report contains all the patient demographics as well as all the parameters of the simulation. It also contains snapshots of the DVH and of sampled cross-sections of the patient where important information is depicted. The user is also able to add as many snapshots of the case as he/she considers appropriate.
This report contains the date and the name of the users that approved and reviewed the simulation.
Dose computation algorithms
One of the first problems faced by our group of professionals working in RADIANCE was that the original water measurements were not an accurate assessment of the dose deposit as heterogeneity of the human body was not considered (5). This led to the development/adaptation of dose calculation algorithms specifically dedicated for IORT such as PB and MC based dose calculation algorithms.
Pencil beam (PB)
The “Pencil Beam” algorithm for electrons implemented in RADIANCE is mainly based on the work of Fermi-Eyges (16). This algorithm takes into account the multiple Coulomb scattering of primary electrons as the primary phenomenon that produces the probability distribution within a material reached by a conveniently collimated narrow electron beam. This multiple scattering has an essentially Gaussian treatment, or at least it can be treated with sufficient accuracy by Gaussian functions. Besides, there are some additional effects which are considered in this implementation such as:
- Stopping radiation production;
- Dispersion of individual electrons on large angles;
- Redistribution of the energy of the beam by means of secondary electrons;
- Widening of the electrons range.
PB implementation included in RADIANCE takes into account all these effects while having a very fast execution (it takes only a few seconds to compute). The limitation of the semi-infinitive layer approach and the poor backscattering modelling (17,18) is covered by the available MC dose computation algorithm.
Monte carlo (MC)
While PB models rely on an analytical description of the total energy distribution released in a semi-infinite volume, MC simulates the fate of individual particles as they propagate through the tissue, based local radiation-matter interactions. MC has been widely used to simulate radiotherapy equipment, dose delivery and radiation sources. In RADIANCE, we have developed a fully parallel reimplementation of the DPM (19) algorithm, which has been optimized for computational efficiency. Dose simulations run indefinitely until the estimated uncertainty is lower than a value specified by the user.
The statistical uncertainty is determined using the history-by-history method (20), which computes the estimated uncertainty σj2 for each voxel as:
where N is the number of simulated histories and Di is the deposited dose per voxel. Periodically during the simulation, the maximum uncertainty is calculated for those voxels whose dose is higher than 50% of the maximum dose (Dmax).
Dose simulations in a water phantom with 1×1×1 mm3 voxels, show (19) that, depending on the applicator nominal energy and chosen applicator diameter, the number of particles needed to meet the 4% uncertainty requirement grows from 4.8 million for a 6 MeV treatment with a 3 cm diameter applicator to 35 million for a 20 MeV treatment with the 9 cm applicator. The simulation time required for these two cases is 15 seconds and 325 seconds respectively.
For electron based IORT we found that, due to the precision of the algorithms in RADIANCE, building a minimal LINAC model for both PB and MC (21,22) algorithms needed just a subset of the measurements needed for the commissioning of the system. Additionally, since these algorithms take into account different tissue densities, it is now possible in RADIANCE to simulate boluses and radio-protections commonly used in the procedure.
Hybrid ray tracing-monte carlo (Hybrid MC)
Our has also developed a hybrid ray tracing—MC algorithm (23) capable of fast and accurate dose calculations with low energy photons. This algorithm has been adapted to the Intrabeam (Carl Zeiss Meditec, Jena, Germany) device. The applicators for this device, as shown in Figure 5, differ from those encountered in electron based IORT in shape and size. In Intrabeam, spherical applicators are used in breast applications, needle applicators are used in vertebral metastases, surface applicators are used for tumors in the surface of the body like skin cancer and flat applicators are used for tumors of surgically exposed surfaces.
Imaging
The proposed planning phases of IORT considering the usage of a TPS are as follows:
- Pre-planning: where several treatment or surgical alternatives can be assessed before surgery. Away from the OR time pressures, RADIANCE can improve the preparation for the real procedure. The feasibility of the procedure or the estimated diameter of the applicator could be evaluated (24);
- Intra-planning: where treatment parameters can be updated from the preplanning simulation during the procedure to assess the impact of intra-surgical modifications;
- Post-planning: where postsurgical control CT studies combined with the simulation tool enable better patient follow-up and also assessment of correlations for late normal tissue toxicity and topographic characteristics of cancer control or relapse.
In any of these phases, the procedure requires an image of the patient that could be used for dose calculation (in case it was CT) or to be used only as reference (MRI, ultrasound, etc.) where the virtual applicator could be manipulated and its position/orientation double checked against the real scenario. In the latter case, since there is no tissue absorption information the dosimetry calculation algorithms would assume that the entire medium is water. Hence RADIANCE can be used with any image modality.
RADIANCE has been used with pre and intra-operative images so far:
Pre-operative images
Preoperative images were used extensively during the inception of RADIANCE. Two institutions were involved in an evaluation study (24) where 14 clinical cases were planned blindly by several ROs with the help of RADIANCE. A high agreement was found in three cases for breast cancer, three rectal cancers, one retroperitoneal sarcoma, one rectal and one ovary relapse. All ORs performed similar tumor and high risk areas segmentation. Simulation parameter agreement was above 83%, and the average applicator position difference was 1.26 cm (0.6-2.19). However, the remaining locations showed higher deviations in the results because of different criteria for contouring high risk areas (one rectal and one pancreas) and different surgical access definition (two rectal and one Ewing).
The results of this study confirmed the assumed benefits of using RADIANCE with preoperative images: guarantees the documentation of the procedure, facilitates the quality assurance as well as boosting the adoption of the technique by reducing the learning curve of the ROs and improves the communication with the surgical team.
Later studies (25-28) have been published confirming the results from the previous validation study and hence, the benefits of the TPS.
Intra-operative images
Although preoperative images have demonstrated good benefit to the IORT planning process, having intra-operative images of the patient however has been repeatedly requested by IORT professionals and identified as the main limitation of IORT 3D planning adoption. Their skepticism was related to the validity of the treatment plan based on images that depicted a different scenario from the real one (except on some localizations such as rectal cases) due to different patient positioning, gravity, breathing, organ displacement, bleeding, etc.
These limitations could be overcome if intraoperative images were available during the IOERT procedure. Ongoing experiments are evaluating the feasibility of acquiring intraoperative CT images of the patients during patient transport and before IOERT irradiation. Unfortunately, this solution would not be adequate if a mobile LINAC is used to deliver the radiation, what is becoming common practice in most hospitals. In this scenario, intraoperative CT devices could be introduced, providing 3D images of the patient to update the dose distribution in real time (29).
Surgical navigation systems
An important alternative/complement to intra-operative imaging is the usage of tracking systems. Image-guided systems would provide guidance by using preoperative and/or intraoperative imaging during the IORT treatment. In reference (30), García-Vázquez et al. proposed a new optical-based navigation system specifically adapted to IOERT procedures. In this work, an optical rigid-body made up of retro-reflective sphere-shaped markers was attached to the applicator tube (Figure 6). Registration between patient and tracking system was performed by using several markers over the patient. Note that this scheme is applicable to either conventional or mobile LINACs.
Exact position of IORT in vivo dosimetry (31) devices could also be identified with the proposed navigated workflow. Hence, device readings could be compared to the dose distribution at such position in the plan.
Although RADIANCE solves part of the simulation necessary for IORT procedures, the real treatment scenario cannot be documented and compared with the pre-planning phase. Intra-planning vs. preplanning and post-planning vs. intra-planning comparisons became a necessity. Therefore, registration techniques allowing image acquisitions are required.
Surgical simulation
Intraoperative Radiotherapy involves surgical and radiotherapy procedures, and so, the pre-planning should cover both simulations.
RADIANCE provides basic surgical simulation tools which allow virtual resection of the tumor or the definition of the surgical frame. With the latter tool, the RO defines the expected anatomic regional access (e.g., lateral, anterior, perineal) and dimensions of the surgical incision. This feature improves the representation of the procedure in the 3D view and at the same time limits the possible movements of the applicator as well as modifying the underlying tissue by assigning it the density of air.
In addition, we are developing new generation surgical simulation tools which will improve user (surgeon) capabilities of planning. This work is carried out jointly by GMV and Universidad Rey Juan Carlos, who already worked successfully in the past to develop an arthroscopy simulator that proved to be a useful tool for surgeons (32). Our first step has been to develop interactive deformation algorithms (33,34) on volumetric data (CT scan) to simulate the effect of putting the organs apart to expose the treatment area. Cutting capabilities and enhanced interactivity of the user interface will follow to this research to complete a basic surgical simulation system.
Automatic segmentation; fusion and registration of medical images
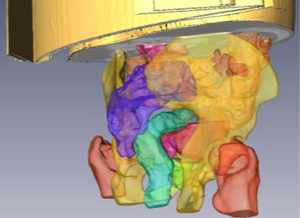
Dose estimations at the tumor bed and at the organs at risk are facilitated thanks to the tools incorporated into the planning system to manually segment any structure or organ of interest. Additionally different automatic segmentation methods, initialized by a seed per organ, have been developed based on morphological reconstruction, graph-cut and level-set methods (35). Figure 7 shows the results of different organs segmented through these methods in an IORT rectum planning procedure.
Additionally multimodal non-rigid alignment techniques have been applied to incorporate information coming from other datasets, such as MRI, into the planning. Methods investigated are mutual information based techniques with a modified metric that reinforces the local statistics of the organ of interest (ref fusion organ focus) (36).
Data exchange standardization
The need of communication of IORT enabled medical devices with other radiotherapy information systems requires the adoption of standards on information exchange formats. The standard associated to radiotherapy is DICOM.RT. Our group is working in the adaption of such standard to cover IORT.
The DICOM standard has been extended to incorporate many medical specialties such as radiation therapy, cardiology, pathology, and ophthalmology. These modifications allow the viewing of images of a patient with special specific information about the procedure. In the radiotherapy field seven DICOM objects have been added to the standard [known as DICOM-RT (37)]. They are RT Image, RT Structure Set, RT Plan, RT Dose, RT Beams Treatment Record, RT Brachy Treatment Record, and RT Treatment Summary Record. These objects set the standard for integration of radiation therapy information within an electronic patient record, which would then ease the interoperability of different radiation therapy systems, enabling sharing of information from different systems.
RADIANCE already implements export capabilities with respect to RT Dose and RT Structure Set objects and import capabilities of DICOM RT Structure Set objects.
With respect to RT Plan, for IORT procedure based on conventional LINACs these existing objects contain the necessary fields to record all the parameters of the procedure, however, for in-OR IORT devices (such as Intrabeam, Mobetron, LIAC) this is not an easy task. It is necessary to find a common way for all vendors to define the minimum number of parameters needed to conglomerate all systems. Some of the areas we are currently proposing are:
- Allow the understanding/communication between RADIANCE and the mobile LINAC by determining a common reference system for all LINACs;
- Allow the dose alignment of mixed treatments, for instance external radiotherapy with IORT;
- Define a common framework for the different degrees of freedom that offer all of the aforementioned mobile LINACs;
- Define a reference system to relate the LINAC’s position against the surgical table and vice versa.
Currently, RADIANCE uses the patient coordinate system to have an independent reference system, and the navigation system converts or transforms from image coordinates to patient coordinates. Once the degrees of freedom of the mobile LINAC are known, then the accelerator will execute the plan and will position the beams in the requested position and orientation, but at the moment RADIANCE assumes that a mobile LINAC has enough degrees of freedom to access the whole patient.
We are in the process of defining a new proposal for RT Plan which will cover these issues.
Dose summation: radiobiology
Once we are able to export dose in the TPS in RT Dose we are capable of summing the dose of IORT plus External Beam Radiation Therapy (EBRT) when both procedures are mixed (boost).
RADIANCE currently exports the dose in physical units, but if we want to sum both doses, having EBRT a fractionated treatment and IORT a single dose, we have to convert the dose into radiobiological equivalence.
The linear-quadratic model (38) has proven to be a valid model for small doses but when we are considering high doses such the ones in IORT, this model needs to be revaluated. For Intrabeam system pilot studies (39) have been done, however further experimental data is needed to provide a basis for biological effect modelling as part of treatment planning for individual patients.
Risk analysis
Finally, with the emergence of imaging tools like those mentioned before and the adoption of RADIANCE as a planning tool in IORT, the implication is that the IORT procedure is changing. These changes need to be analyzed and modification to standards for quality and safety be performed. RADIANCE was used as part of a failure mode and effect analysis (FMEA) (40).
This is the first FMEA analysis for IORT including a TPS. 57 potential modes and effects were identified by our group and classified into ‘treatment cancellation’ and ‘delivering an unintended dose’. They were graded from ‘inconvenience’ or ‘suboptimal treatment’ to ‘total cancellation’ or ‘potentially wrong’ or ‘very wrong administered dose’, although these latter effects have never been experienced in the hospital that carried out the study. Risk priority numbers (RPNs) ranged from 3 to 324 and summed 4,804 in total. Interventions were proposed to circumvent these issues (double checking, interlocking, automation, and structural changes) reducing the final total RPN to 1,320.
The use of a TPS like RADIANCE attempts to provide IORT with the highly technologically developed benchmarks standards of radiotherapy procedures like image guided brachytherapy or external radiotherapy.
Results
Evaluation studies
A preliminary usability study (24) with several use cases was performed with the help of several ROs to demonstrate the validity of the system. Using RADIANCE with preoperative images guarantees the documentation of the procedure, facilitates the quality assurance as well as boosting the adoption of the technique by reducing the learning curve of the ROs and improving the communication with the surgical team.
Hospitals located in seven different countries have been using RADIANCE in their clinical routine or. Results based on feedback from those Institutions are grouped by the localization of the lesions.
Rectal cancer
All Institutions have agreed that rectal cancer is the best case for using RADIANCE with preoperative images because there is little difference, near the volume of interest, with the real case. In fact, those differences are due to organs that are going to be displaced during the procedure and which have been resected from the RADIANCE simulation. As the applicator, most of the times, cannot be in direct contact with the tumor bed, due to the geometry of the cavity, ROs have been able to simulate interfaces of air between the applicator and the tumor bed, leading to make changes about the energy of the accelerator in order to have a more optimized dosage.
Breast cancer
Due to the vast differences between preoperative images and the real surgical scenario, all institutions have concluded that RADIANCE will be more useful when using intraoperative images. Although one Institution reported use of deformed contours around the location of the applicator, although this does not resemble the real deformation, it gives some information about the deposited dose around the area of interest. Still, the ability to simulate the dose distribution, to forecast that some vital organs are receiving high dosage and the capability of recording all relevant information are also part of their feedback.
Retroperitoneal sarcoma
Retroperitoneal sarcomas are complex anatomical targets for postsurgical IORT procedures. Some Institutions have reported doing preplanning, especially for the surgeon and RO to agree on a course of action. Some cases have been chosen as case studies for novel oncologists.
Skin cancer and spinal metastases
Institutions with INTRABEAM machines will be able now to use RADIANCE to simulate the radiation deposit using flat/surface applicators and needle applicator, for skin and spinal metastases, respectively.
Discussion
Our group has been involved in the development of a TPS for IORT procedures. The name of this tool is RADIANCE and it is so far the only TPS available for IORT. Originally developed as a simple tool capable of displaying ideal water based measurements overlaid on a CT image, it has been incorporating new developments capable of bringing IORT planning at the same level as conventional radiotherapy.
We have worked in providing accurate and fast dose calculation algorithms such as PB and MC for its use in IORT cases. Having achieved this, we are now facing the problem of using intraoperative images rather than preoperative ones. We have also introduced the use of surgical navigation in IORT and RADIANCE in particular so that it is possible to have a common reference system for all the elements intervening in the procedure. It is now possible to follow the positioning of the applicator in real time on the screen, allowing a more accurate simulation.
Documentation was one of the things lacking in IORT. Our group has also been working towards adding new information to DICOM-RT objects so that they include all the new parameters required for IORT. So far, RADIANCE generates generic DICOM-RT objects that can be used by other systems but they do not contain information about the degrees of freedom of the mobile accelerator. This is needed to make the mobile LINAC to execute the exact plan.
Using a TPS within IORT implies a new analysis of the risks involved in the procedure. We have now analyzed the new risks and implemented some processes to prevent them for IOERT. We will be working in applying the same scheme to low energy protons.
RADIANCE is being used clinically in many centers around the world and it is proven to have an impact on the quality and accuracy of IORT procedures. Many procedures in different localizations have been planned with RADIANCE such as breast cancer, rectal cancer, retroperitoneal sarcomas, pancreatic cancer, ovarian and now skin cancer with INTRABEAM machines, the reported cases from the Institutions that have adopted RADIANCE are promising. There are still many issues to tackle but the use of RADIANCE is bringing standardization to IORT.
Acknowledgments
We would like to give a special and emotive acknowledgement and tribute to Prof. Juan Antonio Santos Miranda. Without his selfless exhausting work and brilliant knowledge this project would have never been accomplished.
Funding: This work was supported by the Spanish Ministry of Science and Innovation, currently integrated in the Ministry of Economy, under grants PSE-300000-2009-5, IPT-300000-2010-003, IPT-2012-0401-300000, TEC2010-21619-C04, PI08/90473, PI09/90628, PI11/01659, PI11/02908, and FPA2010-17142, by CDTI CENIT Program (AMIT Project), and by Comunidad de Madrid grant ARTEMIS SP2009/DPI-1802. We also acknowledge the European Union for their funding through the European Fund for Regional Development (ERDF).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frank A. Giordano, Pedro Carlos Lara and Frederik Wenz) for the series “Intraoperative Radiotherapy II” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Carlos Illana, Manlio F. Valdivieso, Raúl Rodríguez and Samuel Rodríguez work for the company which develops RADIANCE TPS. Drs. Calvo, Desco and Pascau are co-inventors in a registered patent together with GMV. The authors declare no conflict of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gunderson L, Willett C, Harrison L, et al. eds. Intraoperative irradiation: Techniques and Results. New York: Humana Press, 2011.
- Calvo FA, Meirino RM, Orecchia R. Intraoperative radiation therapy first part: rationale and techniques. Crit Rev Oncol Hematol 2006;59:106-15. [PubMed]
- Krengli M, Sedlmayer F, Calvo F, et al. ISIORT pooled analysis 2013 update: clinical and technical characteristics of intraoperative radiotherapy. Transl Cancer Res 2014;3:48-58.
- Sole CV, Calvo FA, Ferrer C, et al. Bibliometrics of intraoperative radiotherapy: analysis of technology, practice and publication tendencies. Strahlenther Onkol 2014;190:1111-6. [PubMed]
- Beddar AS, Biggs PJ, Chang S, et al. Intraoperative radiation therapy using mobile electron linear accelerators: report of AAPM Radiation Therapy Committee Task Group No. 72. Med Phys 2006;33:1476-89. [PubMed]
- Medical Exposure Directive (MED). COUNCIL DIRECTIVE 97/43/EURATOM of 30 June 1997. Available online: http://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:31997L0043
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [PubMed]
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Rosi A, Viti V. Istituto Superiore di Sanità Guidelines for intra-operative radiation therapy. 2003, ISTISAN 03/1 IT.
- Ma CM, Coffey CW, DeWerd LA, et al. AAPM protocol for 40-300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Med Phys 2001;28:868-93. [PubMed]
- Nag S, Martinez-Monge R, Nieroda C, et al. Radioimmunoguided-intraoperative radiation therapy in colorectal carcinoma: a new technique to precisely define the clinical target volume. Int J Radiat Oncol Biol Phys 1999;44:133-7. [PubMed]
- Prescribing, recording, and reporting electron beam therapy. ICRU report no. 71. Oxford: Oxford University Press, 2004. ISBN 0198566786. Available online: http://jicru.oxfordjournals.org/content/4/1/5.full
- Digital Imaging and Communications in Medicine (DICOM) Supplement 11 Radiotherapy Objects, Final Text, NEMA Standards Publication, 1997.
- Desco M, López J, Calvo FA, et al. Simulated surgery on computed tomography and magnetic resonance images: an aid for intraoperative radiotherapy. Comput Aided Surg 1997;2:333-9. [PubMed]
- Hogstrom KR, Mills MD, Almond PR. Electron beam dose calculations. Phys Med Biol 1981;26:445-59. [PubMed]
- López-Tarjuelo1 J, Lardiés M, García-Romero A, et al. Pencil Beam for Electron Intraoperative Radiotherapy. Early Results From Profile and Percentage Depth Dose Modelling. Med Phys 2010;37:3206.
- Calama Santiago JA, Garcia-Romero A, Lardiés Fleta D, et al. 25 poster Pencil Beam for electron intraoperative radiotherapy. Results of dose calculations in heterogeneous media. Radiother Oncol 2011;99:S13.
- Guerra P, Udías JM, Herranz E, et al. Feasibility assessment of the interactive use of a Monte Carlo algorithm in treatment planning for intraoperative electron radiation therapy. Phys Med Biol 2014;59:7159-79. [PubMed]
- Walters BR, Kawrakow I, Rogers DW. History by history statistical estimators in the BEAM code system. Med Phys 2002;29:2745-52. [PubMed]
- Ibáñez P, Vidal M, García-Marcos R, et al. Validation of a phase space determination algorithm for intraoperative radiation therapy. Radiother Oncol 2014;111:331.
- Herranz E, Herraiz JL, Ibáñez P, et al. Phase space determination from measured dose data for intraoperative electron radiation therapy. Phys Med Biol 2015;60:375-401. [PubMed]
- Vidal M, Ibáñez P, Cal González J, et al. Hybrid Monte Carlo dose algorithm for low energy X-rays intraoperative radiation therapy. Radiother Oncol 2014;111:117-8.
- Pascau J, Santos Miranda JA, Calvo FA, et al. An innovative tool for intraoperative electron beam radiotherapy simulation and planning: description and initial evaluation by radiation oncologists. Int J Radiat Oncol Biol Phys 2012;83:e287-95. [PubMed]
- Calvo FA, Villanueva-Martinez J, Sallabanda M, et al. Imaging in treatment planning opportunities for intraoperative electron irradiation (IOERT): developments in the context of radiance system. Accepted for publication. Rep Pract Oncol Radiother 2013;19:239-45.
- Calvo FA, Sole CV, González ME, et al. Research opportunities in intraoperative radiation therapy: the next decade 2013-2023. Clin Transl Oncol 2013;15:683-90. [PubMed]
- Calvo FA, Sallabanda M, Sole CV, et al. Intraoperative radiation therapy opportunities for clinical practice normalization: Data recording and innovative development. Rep Pract Oncol Radiother 2013;19:246-52. [PubMed]
- Calvo F, Sole C, Herranz R, et al. Intraoperative radiotherapy with electrons: fundamentals, results, and innovation. Ecancermedicalscience 2013;7:339. [PubMed]
- Siewerdsen JH. Cone-Beam CT with a Flat-Panel Detector: From Image Science to Image-Guided Surgery. Nucl Instrum Methods Phys Res A 2011;648:S241-S250. [PubMed]
- García-Vázquez V, Marinetto E, Santos-Miranda JA, et al. Feasibility of integrating a multi-camera optical tracking system in intra-operative electron radiation therapy scenarios. Phys Med Biol 2013;58:8769-82. [PubMed]
- López-Tarjuelo J, Bouché-Babiloni A, Morillo-Macías V, et al. In vivo dosimetry in intraoperative electron radiotherapy: microMOSFETs, radiochromic films and a general-purpose linac. Strahlenther Onkol 2014;190:1060-5. [PubMed]
- Andersen C, Winding TN, Vesterby MS. Development of simulated arthroscopic skills. Acta Orthop 2011;82:90-5. [PubMed]
- Gascón J, Espadero J. Pérez A, et al. Fast deformation of volume data using tetrahedral mesh rasterization. In: Proc. of the ACM SIGGRAPH/Eurographics Symposium on Computer Animation 2013:181-5.
- Torres R, Espadero JM, Calvo F, et al. eds. Interactive Deformation of Heterogeneous Volume Data. Biomedical Simulation - 6th International Symposium, ISBMS 2014, Strasbourg, 2014.
- Jimenez-Carretero D, Fernandez-de-Manuel L, Pascau J, et al. Optimal Multiresolution 3D Level-Set Method for Liver Segmentation incorporating Local Curvature Constraints". Proc 33rd Annual International IEEE EMBS Conference. Boston, USA, 2011:3419-22.
- Fernandez-de-Manuel L, Wollny G, Kybic J, et al. Organ-focused mutual information for nonrigid multimodal registration of liver CT and Gd-EOB-DTPA-enhanced MRI. Med Image Anal 2014;18:22-35. [PubMed]
- Law MY, Liu B. Informatics in radiology: DICOM-RT and its utilization in radiation therapy. Radiographics 2009;29:655-67. [PubMed]
- Douglas BG, Fowler JF. The effect of multiple small doses of x rays on skin reactions in the mouse and a basic interpretation. Radiat Res 1976;66:401-26. [PubMed]
- Herskind C, Steil V, Kraus-Tiefenbacher U, et al. Radiobiological aspects of intraoperative radiotherapy (IORT) with isotropic low-energy X rays for early-stage breast cancer. Radiat Res 2005;163:208-15. [PubMed]
- López-Tarjuelo J. Failure mode and effect analysis oriented to risk-reduction interventions in intraoperative electron radiation therapy: The specific impact of patient transportation, automation, and treatment planning availability. Radiother Oncol 2014;113:283-289. [PubMed]