
Intraoperative radiotherapy in locally-advanced and recurrent rectal cancer: retrospective review of 68 cases
Introduction
Despite the advances made in recent years, treatment of advanced rectal cancer remains challenging. Although complete surgical excision of the tumour and margins is the cornerstone of treatment, most patients require a multimodal approach involving surgery, chemotherapy, and external beam radiotherapy (EBRT) (1-4). However, even with the best treatment, the risk of recurrence in locally-advanced rectal cancer remains high (up to 40%) (5). For this reason, new approaches are needed to prevent recurrence in high risk cases. One such approach is the application of intraoperative radio therapy (IORT).
In IORT, a precise dose of radiation (typically 10-20 Gy) is delivered to the tumour bed immediately after resection (6). The therapeutic advantage of this approach is that the anatomical region considered to present a high risk of recurrence can be targeted directly while adjacent healthy tissues and structures can be shifted out of the radiation field, or shielded during the procedure. Although recent randomised controlled trials (RCTs) have confirmed the benefits of IORT for breast cancer (7), only a few RCTs have evaluated the use of this modality in locally-advanced and recurrent rectal cancer (8,9).
In recent years, the emergence of compact, mobile devices equipped with radiation protection has enabled the expansion of IORT for use in a wide variety of tumour types (8,10). Among these latest-generation devices is the photon radiosurgery system (PRS), a miniature X-ray radiation device most commonly used for IORT in breast cancer [5]. Despite the widespread use of this device in breast cancer, only one study has assessed its application in rectal cancer (11).
In this context, we present our initial experience with the PRS system as part of the multimodal treatment of rectal cancer. The main aim was to assess short and medium-term outcomes in patients with primary rectal cancer (PRC) or recurrent rectal cancer (RRC) treated with surgery and IORT.
Patients and methods
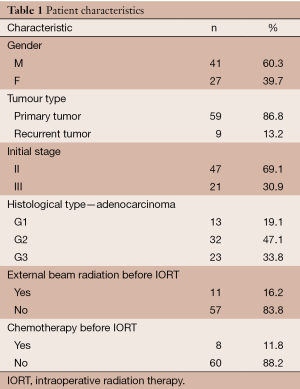
We retrospectively evaluated a total of 68 pts (41 men, 27 women) who underwent radical surgery and IORT at our centre (Clinical Oncological Center, Krasnodar, Russia) from December 2012 to December 2014 (Table 1). Eligible patients were those with a diagnosis of T3-4 rectal cancer or pelvic recurrence who underwent radical surgery and were considered to have a high risk of positive resection margins. Patients with distant metastasis were excluded.
Full table
Median patient age was 67 (range, 33-82) years and median body mass index (BMI) was 28.1 (range, 19.5-44.3). Most patients were diagnosed with stage II (47 cases) or stage III (21 cases) PRC. The nine of this patients with recurrent tumours were staged according to the Wanebo system (12), as follows: Tr3 (6 cases), Tr4 (2 cases), and Tr5 (1 case).
Surgery was carried out according to standard protocols. Patients with PRC underwent an anterior rectal resection (ARR) with total mesorectal excision (TME) (39 cases). In 16 cases, an abdominoperineal extirpation of the rectum was performed. In four cases, the resection used the abdomino-anal approach. The surgical approach for 9 pts with RRC involved abdominal or combined abdominoperineal or abdominoanal resection; in three cases, nearby organs were also resected.
After specimen excision, we performed a careful macroscopic assessment to determine and mark the area with the highest risk of involvement. A circular border surrounding the resected tumour margins was marked to estimate the radiation field needed. For IORT delivery, the INTRABEAM® PRS (Carl Zeiss Meditec, Oberkochen, Germany) was used. In all cases, IORT was performed immediately following tumour resection. In most cases (61 pts), we used a 5 cm removable spherical applicator; in the remaining patients (seven cases), a 4.5 cm applicator was used. The small intestine was covered with gauze and moved in the cranial direction using an extractor; in certain cases, tourniquets were applied to the ureters and these were separated laterally from the radiation field. The vascular fascicles and ureters were protected with special sterile plates and dry gauze stacked on the pelvic sidewalls. In patients who underwent ARR, the rectal stump was protected in the same way. A single fraction of radiation was delivered with the assistance of a medical physicist and a radiologist. Afterwards, the surgical intervention continued. For sphincter-sparing operations, an anastomosis was created. In the abdominal perineal resection, the perineal wound was closed. In all cases with an anastomosis, preventive ileostomy was performed.
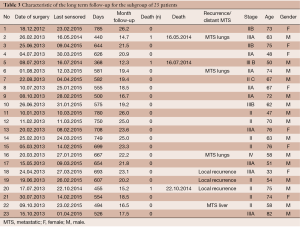
Due to the relatively short follow-up for the patients treated in the year 2014, a subgroup analysis involving only patients treated in 2012 or 2013 was performed. A total of 23 cases were included in this subgroup analysis, with a median follow-up of 20.7 (range, 17-28) months.
Statistical analysis
Cumulative, overall, and recurrence-free survival (RFS) rates were calculated with the Kaplan-Meier method.
Results
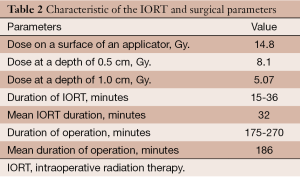
A dose of 5.07 Gy was prescribed to a depth of 1 cm. The median dose on the applicator surface was 14.8 (range, 8.39-17) Gy. The median duration of the IORT sessions was 31.9 (range, 15-36) minutes (Table 2). No radiation-related events or complications of note were observed in the postoperative period. Mean overall hospitalization time (including pre-operative admission) was 21.2 (range, 11-33) days. Postoperatively, the mean hospital stay was 17.7 (range, 9-25) days.
Full table
Postoperative infections were observed in 3 pts, as follows: abdominal wound infection (2 cases), and perineal wound infection (1 case). The overall complication rate for all 68 pts was 4.4%. An atonic bladder occurred in one case. No cases of colorectal anastomosis leakage were recorded.
Of the 23 cases included in the subgroup analysis, 6 received neoadjuvant chemo-radiotherapy due to pelvic recurrence and 18 received 3 to 6 cycles of adjuvant chemotherapy (Fluorouracil and Levamisole).
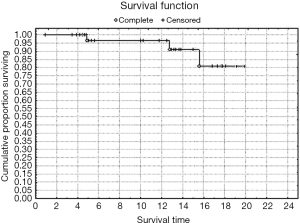
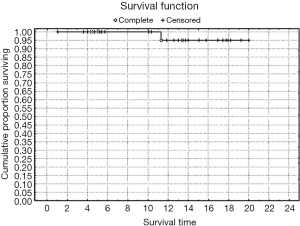
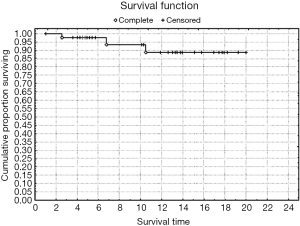
Rates of both overall survival (OS) and local RFS in this 23-patient subgroup were 87% [standard error (SE) =6.3] (Figures 1,2, Table 3). Distant metastasis was observed in 4 pts (lung, 3 cases; liver, 1 case); as a result, the distant metastasis-free survival rate was 82.6% (SE=6.5) (Figure 3). No unusual complications (e.g., fibrosis of the ureter, hydronephrosis, neuropathy of the lower extremities) were observed.
Full table
Discussion
In this study, we evaluated treatment outcomes in a group of patients with rectal cancer who underwent multimodal treatment including radical surgery and IORT delivered with the INTRABEAM® PRS. To our knowledge, this is only the second study to evaluate this system in colorectal cancer. As our results show, both OS and RFS in the subgroup with long follow-up was quite good and comparable to similar studies that used electron IORT (9,13). Moreover, few complications were reported and patients did not require extended hospitalization beyond the usual time for such surgeries.
In rectal cancer, IORT is typically delivered with electrons (8,14-17) or, less frequently, with high-dose rate brachytherapy (HDR BT) (18,19). To date, the only other study apart from ours to use the Intrabeam system to deliver IORT in advanced rectal cancer was the study performed by Guo et al. at the Cleveland Clinic (11). In that study, the authors retrospectively evaluated the results of 42 pts treated for RRC [32] or PRC [10] rectal cancer. All patients underwent radical surgery with a 5 Gy dose to the tumour bed delivered by IORT, calculated for depth of 1 cm. In contrast to our experience, they used a wider range of spherical applicators (from 2 to 5 cm in diameter). The overall 3-year survival rate for RRC and PRC was 43% and 65%, respectively. The 1-year recurrence rate was 16%, and distant metastasis occurred in 32% of the whole cohort. Outcomes in our 23-patient subgroup compare favourably with those reported by Guo and colleagues. As noted, at nearly 21 months of follow-up, OS and local RFS were both 87%. However, given the longer follow-up in that study, it is reasonable to expect that, over time, our results will tend to converge with the outcomes reported by those authors. In addition, in contrast to Guo et al., the bulk of our patients had PRC (rather than RRC), and this difference, together with our shorter follow-up, assuredly explains much of the survival difference between the two studies.
Some authors have suggested that IORT may not provide any additional benefit in locally-advanced rectal cancer (8,15). However, numerous studies have shown that the inclusion of IORT as part of a multimodal treatment approach improves local control in patients with microscopically-involved circumferential resection margins (CRM) (14,16,17,20,21). In a recent study, Alberda et al. found that patients with a microscopically-involved CRM treated with IORT had a significantly better cumulative 5-year local RFS vs. patients treated without IORT (84 vs. 41%, P=0.01). Moreover, on the multivariate analysis, IORT was independently associated with a lower rate of local recurrence. In contrast, another recent study (22) suggested that IORT may improve outcomes regardless of microscopic margin status. These data suggest that IORT may be indicated in tumours with close or positive microscopic margins.
Many studies of electron IORT have reported serious complications, including intestinal fistulas, sacral necrosis, post-radiation ureter damage (fibrosis), and hydronephrosis (9,14,23,24). In contrast, we did not observe any serious complications, nor did Guo et al. (11). These findings, while still preliminary, suggest that the Intrabeam system has a good safety profile, as has been previously demonstrated in other cancer localizations (25). Another important safety advantage of the PRS system is the high degree of radiation safety that it affords the surgical team and its ease of manoeuvrability, which allows the radiation source to be precisely positioned near the tumour bed. Schneider et al. (26) also highlighted an important benefit of using low kV X-ray IORT in comparison to HDR BT or high-energy elections. Compared to those modalities, which require substantial radiation protection measures (including a shielded operating room), low kV X-ray systems like the Intrabeam PRS system require only minimal radiation protection measures, making it both safer and easier for the surgical team.
In general, the results presented here confirm the conclusions reached by Guo et al. regarding the safety and effectiveness of the PRS INTRABEAM® system (11). As those authors note, because the dose-rate of this device is lower than other modalities, treatment delivery times are longer (median duration of IORT was 35 minutes in their series and 32 minutes in ours). However, this is a non-critical increase in treatment duration versus electron IORT. Likewise, we found that the hospitalization time did not increase when compared to non-IORT treatment.
Limitations
The most obvious limitations of this study are its retrospective design and lack of a control group. In addition, follow-up for the entire group is relatively short; however, to mitigate that issue, we performed a subgroup analysis of the 23 pts with the longest follow-up (up to 28 months).
Conclusions
The initial results presented here suggest that the Intrabeam PRS is a safe technology for use in IORT in the multimodal treatment of rectal cancer. The Intrabeam PRS requires a small but non-critical increase in operating time. No specific complications related to this system were observed. Based on these findings and considering that the Intrabeam system is already widely-used in other cancer types, we believe that this method can be safely and confidently integrated into the multimodal treatment algorithms for rectal cancer at specialized cancer care institutions. However, future studies are needed to report long term results of this system in rectal cancer.
Acknowledgments
Abstract and poster presentation was accepted on 7th European Multidisciplinary Colorectal Cancer Congress, 23-25 November 2014, Netherlands, Amsterdam. We wish to thank Bradleyl Londres for his editorial assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frank A. Giordano, Pedro Carlos Lara and Frederik Wenz) for the series “Intraoperative Radiotherapy II” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.04.07). The series “Intraoperative Radiotherapy II” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board. Written informed consent was obtained from every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yeung JM, Ngan S, Lynch C, et al. Intraoperative radiotherapy and colorectal cancer. Minerva Chir 2010;65:161-71. [PubMed]
- Rodriguez-Bigas MA, Chang GJ, Skibber JM. Multidisciplinary approach to recurrent/unresectable rectal cancer: how to prepare for the extent of resection. Surg Oncol Clin N Am 2010;19:847-59. [PubMed]
- Wong RK, Tandan V, De Silva S, et al. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev 2007;CD002102 [PubMed]
- Petersen SH, Harling H, Kirkeby LT, et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012;3:CD004078 [PubMed]
- Mirnezami R, Chang GJ, Das P, et al. Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg Oncol 2013;22:22-35. [PubMed]
- Gunderson LL. Rationale for and results of intraoperative radiation therapy. Cancer 1994;74:537-41. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Dubois JB, Bussieres E, Richaud P, et al. Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study. Radiother Oncol 2011;98:298-303. [PubMed]
- Dresen RC, Gosens MJ, Martijn H, et al. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol 2008;15:1937-47. [PubMed]
- Debenham BJ, Hu KS, Harrison LB. Present status and future directions of intraoperative radiotherapy. Lancet Oncol 2013;14:e457-64. [PubMed]
- Guo S, Reddy CA, Kolar M, et al. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol 2012;7:110. [PubMed]
- Wanebo HJ, Antoniuk P, Koness RJ, et al. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum 1999;42:1438-48. [PubMed]
- Haddock MG, Miller RC, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 2011;79:143-50. [PubMed]
- Ferenschild FT, Vermaas M, Nuyttens JJ, et al. Value of intraoperative radiotherapy in locally advanced rectal cancer. Dis Colon Rectum 2006;49:1257-65. [PubMed]
- Masaki T, Takayama M, Matsuoka H, et al. Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer. Langenbecks Arch Surg 2008;393:173-80. [PubMed]
- Kusters M, Valentini V, Calvo FA, et al. Results of European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol 2010;21:1279-84. [PubMed]
- Valentini V, Coco C, Rizzo G, et al. Outcomes of clinical T4M0 extra-peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery: a prospective evaluation of a single institutional experience. Surgery 2009;145:486-94. [PubMed]
- Vermaas M, Nuyttens JJ, Ferenschild FT, et al. Reirradiation, surgery and IORT for recurrent rectal cancer in previously irradiated patients. Radiother Oncol 2008;87:357-60. [PubMed]
- Martínez-Monge R, Nag S, Martin EW. Three different intraoperative radiation modalities (electron beam, high-dose-rate brachytherapy, and iodine-125 brachytherapy) in the adjuvant treatment of patients with recurrent colorectal adenocarcinoma. Cancer 1999;86:236-47. [PubMed]
- Ratto C, Valentini V, Morganti AG, et al. Combined-modality therapy in locally advanced primary rectal cancer. Dis Colon Rectum 2003;46:59-67. [PubMed]
- Alberda WJ, Verhoef C, Nuyttens JJ, et al. Intraoperative radiation therapy reduces local recurrence rates in patients with microscopically involved circumferential resection margins after resection of locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2014;88:1032-40. [PubMed]
- Hyngstrom JR, Tzeng CW, Beddar S, et al. Intraoperative radiation therapy for locally advanced primary and recurrent colorectal cancer: ten-year institutional experience. J Surg Oncol 2014;109:652-8. [PubMed]
- Eble MJ, Lehnert T, Treiber M, et al. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol 1998;49:169-74. [PubMed]
- Nuyttens JJ, Kolkman-Deurloo IK, Vermaas M, et al. High-dose-rate intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2004;58:106-12. [PubMed]
- Grobmyer SR, Lightsey JL, Bryant CM, et al. Low-kilovoltage, single-dose intraoperative radiation therapy for breast cancer: results and impact on a multidisciplinary breast cancer program. J Am Coll Surg 2013;216:617-23; discussion 623-4. [PubMed]
- Schneider F, Clausen S, Thölking J, et al. A novel approach for superficial intraoperative radiotherapy (IORT) using a 50 kV X-ray source: a technical and case report. J Appl Clin Med Phys 2014;15:4502. [PubMed]


