
EGFR and HER2: is there a role in ovarian cancer?
Introduction
Ovarian cancer is the leading cause of death from gynecologic malignancies. Although conventional chemotherapy and surgery for advanced ovarian cancer have improved over the years with better outcomes, the majority of women still die with drug-resistant disease and as such, there is a critical need for the development of molecular targeted therapies (1). Anti-angiogenesis therapies, such as bevacizumab, and poly ADP ribose polymerase (PARP) inhibitors, have shown substantial anti-tumor activity in ovarian cancer (1). Given positive preclinical data, much interest has been dedicated to studying the ErbB signaling factor pathway in ovarian cancer (1). The ERbB family of receptor tyrosine kinases has a role in the tumorigenesis of many types of solid tumors and consists of the epidermal growth factor receptor (EGFR) (also known as HER1/ErbB1), human EGFR2 (HER2/neu)/ERbB2, HER3/ErbB3 and HER4/ErbB4 (2). The four HER receptors have a key role in cancer and promote tumorigenesis via cell proliferation, survival, migration, adhesion, and differentiation. Each receptor is a type I transmembrane protein consisting of a heavily glycosylated ectodomain, which contains a ligand binding site, an intracellular protein-tyrosine kinase catalytic domain, and a tyrosine-containing cytoplasmic tail. Post receptor signaling by activated HERs include four representative pathways: the Ras-Raf/mitogen activated protein kinase (MAPK) and signal transducer and activation of transcription (STAT) pathways that regulate cell proliferation and differentiation, the phosphoinositidyl-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway that is important for cell survival, and the phospholipase Cγ (PLCγ) pathway that controls calcium-dependent events. Mutations, gene amplifications, and protein overexpression of the HER family members are linked to carcinogenesis (2,3). Overexpression and/or mutations of EGFR and HER2 are well documented in a variety of solid tumors, including ovarian cancer, and have therapeutic implications (4,5). This review will discuss the clinical trials of anti-EGFR and HER2-directed therapies in ovarian cancer, which unfortunately have been largely disappointing and will review mechanisms of resistance to these targeted therapies and future directions.
EGFR in ovarian cancer
The epithelial lining of the ovary normally has weak EGFR expression and in epithelial ovarian carcinoma, EGFR overexpression ranges anywhere from 4-100% of cases (3,6). It has not been consistently shown to correlate with disease aggressiveness; however, expression is associated with poor prognosis and decreased therapeutic responsiveness, which has led to clinical trials of EGFR inhibitors in this disease (3,7-9).
Clinical trials
Small molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies are currently used to block EGFR activity and have been studied in ovarian cancer (Table 1). The most common TKI, erlotinib, is an inhibitor of HER1/EGFR. It is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. It is an orally active, potent, selective inhibitor of the EGFR tyrosine kinase. It reversibly binds to the adenosine triphosphate (ATP)-binding site of EGFR and completely inhibits autophosphorylation by EGFR tyrosine kinase. This results in blockage of downstream EGFR signal-transduction pathways, cell-cycle arrest, and inhibition of angiogenesis (6). There have been several trials with erlotinib in ovarian cancer.
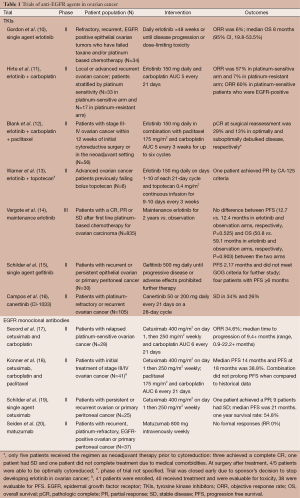
Full table
One of the earliest trials targeting EGFR in ovarian cancer was a phase II study by Gordon et al., which evaluated single agent erlotinib in 34 patients with refractory, recurrent, EGFR positive epithelial ovarian tumors who had failed taxane and/or platinum-based chemotherapy. Patients received daily erlotinib for up to 48 weeks or until disease progression or dose-limiting toxicity. The objective response rate (ORR) was 6% (95% CI, 0.7-19.7%) and the median overall survival (OS) was 8 months (95% CI, 19.8-53.5%). Notably, patients with a rash survived significantly longer than those without a rash (P=0.009) (10). Hirte et al. enrolled 50 patients with local or advanced recurrent ovarian cancer, stratified by platinum sensitivity (n=33 in platinum sensitive arm and n=17 in platinum resistant arm), in a phase II study of erlotinib and carboplatin. Patients were treated with erlotinib 150 mg daily and carboplatin AUC 5 every 21 days. The ORR was 57% in the platinum-sensitive arm and 7% in the platinum-resistant arm. A total of 71% of archival tumors stained positive for EGFR and in platinum-sensitive patients with EGFR-positive tumors, there were 12 responses (60% ORR) and the responding platinum-resistant patient was also EGFR positive. The addition of erlotinib was well tolerated; however, the addition of erlotinib could not reverse platinum-resistance (11). Erlotinib was combined with carboplatin and paclitaxel in a phase II study in the first-line treatment of ovarian cancer. A total of 56 patients were enrolled and 36 patients completed six cycles of the regimen. The primary endpoint, pathologic complete response (pCR) at surgical reassessment, was 29% and 13% in optimally and suboptimally debulked disease, respectively. The primary objective of increasing pCR by two-fold when compared to historical data was not met and EGFR gene amplification was not associated with response rate (12). A small study of six patients previously failing bolus topotecan evaluated continuous infusion topotecan in combination with erlotinib. One patient achieved a partial response (PR) by CA-125 criteria (13).
Recently, Vergote et al. evaluated the efficacy of maintenance erlotinib in a phase III trial of patients with a CR, PR, or stable disease (SD) after first line platinum-based chemotherapy for ovarian carcinoma. A total of 835 patients were randomly assigned to receive maintenance erlotinib for two years or to observation. In an intention-to-treat analysis, the progression free survival (PFS) and OS were similar between the two groups and this study showed no benefit of maintenance erlotinib when compared with standard management. PFS was 12.7 and 12.4 months in the erlotinib and observation arms, respectively (HR adjusted for stratification factors, 1.05; 95% CI, 0.90-1.23; P=0.525). OS was 50.8 and 59.1 months for the erlotinib and observation arms, respectively (HR, 0.99; 95% CI, 0.81-1.20; P=0.903). Only 25.8% of patients in the erlotinib arm stopped treatment due to toxicity and 50.1% required dose modification mainly due to diarrhea or rash. There was no difference in subgroup analyses and quality of life scores were in favor of the observation arm (P=0.0102). Archival tumor tissue was used to evaluate EGFR overexpression by immunohistochemistry (IHC), EGFR copy number by fluorescence in situ hybridization (FISH) and perform EGFR mutation analyses. The 41/248 patients (32.8%) in the erlotinib arm and 49/248 patients (39.8%) in the observation arm demonstrated EGFR positivity. There was no correlation identified between EGFR staining and any of the clinicopathologic variables. In the erlotinib arm, there was no association between PFS, OS, and the IHC staining or FISH score. In the entire cohort, patients who were positive by FISH did have a worse survival than those who were negative for FISH (46.1 vs. 67 months; HR, 1.56; 95% CI, 1.01-2.40; P=0.044). This was similarly seen in a PFS analysis: patients with EGFR positivity by FISH had a shorter PFS than those who were negative (9.6 vs. 16.1 months; HR, 1.57; 95% CI, 1.11-2.22; P=0.01). In DNA mutation analysis performed in 318 patients, the following mutations were demonstrated: EGFR (n=3); KRAS (n=9); NRAS (n=2); BRAF (n=2); and PIK3CA (n=12). In patients with a mutation, the PFS was longer than in those without a mutation (34 vs. 12.2 months, HR, 0.49, 95% CI, 0.28-0.88, P=0.015); however, EGFR-related mutations did not predict for efficacy of erlotinib in the treatment arm (14).
The gynecologic oncology group (GOG) has performed a series of single agent biologic agent trials in persistent or recurrent ovarian or primary peritoneal cancer and has studied gefitinib, another EGFR TKI, in their ‘biologic queue’. However, there was minimal activity in 30 patients with recurrent or persistent epithelial ovarian or primary peritoneal carcinoma treated with gefitinib. Only four patients experienced a PFS of ≥6 months. The median PFS, 2.17 months, did not meet the GOG criteria for further study. A total of 42% of tumors demonstrated EGFR positivity (designated 1+ or higher by IHC) and the four patients with a prolonged PFS had tumors with some EGFR positivity. EGFR mutation analysis was performed in 25 specimens; one EGFR mutation was detected and interestingly, was in the one patient who experienced a PR (15). Another GOG biologics study evaluated canertinib (CI-1033), a 4-anilinoquinazoline that acts irreversibly at the ATP binding site of the ErbB receptor family member. There was a median PFS of 2.2 months, no CR or PRs were observed and there was no relationship between tumor expression of any of the ErbB subtypes, disease stabilization or OS (16). Lapatinib, a dual EGFR and HER2 inhibitor, will be described later in the article.
In addition to studies with EGFR TKIs, there have been studies with an anti EGFR monoclonal antibody, cetuximab (Table 1). Cetuximab and carboplatin was evaluated in 28 patients with relapsed platinum-sensitive ovarian cancer with an ORR of 34.6% and a median time to progression of 9.4+ months (range, 0.9-22.2+ months). Archival tissue for EGFR expression by IHC showed EGFR positivity (≥1%) in 26/28 patients and interestingly, the two patients negative for EGFR both responded to cetuximab. Also of note, the response rates for patients with EGFR positive tumors were 60%, 40% and 33% for 1+, 2+ and 3+ EGFR staining, respectively. The intensity of EGFR staining was not predictive of response and it was hypothesized that high intensity may actually predict for resistance to cetuximab (17). In another phase II study of 40 patients receiving carboplatin, paclitaxel and cetuximab in the initial treatment of stage III/IV ovarian cancer, the median PFS was 14.4 months and PFS at 18 months was 38.8%. The combination was well tolerated but there was no prolongation of PFS when compared to historical data (18). In a phase II trial of single agent cetuximab in 25 patients with persistent or recurrent ovarian or primary peritoneal cancer, one patient achieved a PR, nine patients had SD, and median PFS was 21 months. This trial did not achieve the required minimal activity for further study by the GOG (19). A phase II study of EMD72000 (matuzumab), a humanized anti-EGFR monoclonal antibody, of 37 heavily pre-treated platinum-resistant patients did not yield any responses (20).
HER2 in ovarian cancer
In addition to EGFR, there has been a lot of interest in investigating HER2 in ovarian cancer but studies have been disappointing. HER2 expression in epithelial ovarian cancer is more commonly seen in the serous subtype, in older patients, patients with advanced stage and high-grade differentiation (21). Similarly to EGFR, the rates of HER2 overexpression and/or amplification in ovarian cancer are variable, ranging from 2% to 66% (21,22). HER2 has been studied as a prognostic factor but with contradictory results. While some studies have shown that HER2 expression is associated with a worse prognosis, others have not demonstrated any relationship between HER2 and survival (23-28). There are several anti-HER2 agents that have been approved for breast cancer which have been investigated in ovarian cancer as well: trastuzumab, a humanized monoclonal anti-HER2 antibody, pertuzumab, a recombinant, humanized monoclonal antibody directed against HER2 that inhibits ligand-activated heterodimerization with other HER2 receptors, especially HER3, and lapatinib, a small molecular dual TKI of HER2 and EGFR (29,30).
Clinical trials
Several clinical trials have evaluated anti-HER2 therapies in ovarian cancer (Table 2). GOG 160 evaluated single agent trastuzumab in patients with recurrent or persistent ovarian or primary peritoneal carcinoma with 2+ or 3+ HER2 IHC expression. Out of 837 tumors screened, only 95 (11.4%) showed 2+ or 3+ expression; ultimately, 41 eligible and assessable patients were treated with trastuzumab. ORR was 7.3% and an additional 16 patients (39%) were found to have SD with three receiving therapy for over a year. There was no relationship identified between tumor expression of HER2 and clinical response, PFS, or OS (31).
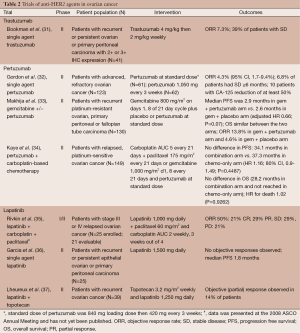
Full table
Gordon et al. evaluated pertuzumab in a phase II, multicenter trial in advanced, refractory ovarian cancer. A total of 61 patients received a loading dose of 840 mg of pertuzumab followed by 420 mg every 3 weeks and 62 patients received 1,050 mg every 3 weeks. The primary endpoint, response rate, was 4.3% (95% CI, 1.7-9.4%). About 6.8% of patients had SD lasting ≥6 months and ten patients had a CA-125 reduction of at least 50%. Tumor biopsies were obtained to assay for HER2 overexpression, amplification and phosphorylated HER2 (pHER2). A total of 28.6% of the biopsies were pHER2-positive by ELISA without amplification and interestingly, these patients had a better outcome following pertuzumab therapy, compared to patients who were pHER2-negative (32). Pertuzumab was then studied in combination with gemcitabine in patients with recurrent platinum-resistant ovarian, primary peritoneal or fallopian tube carcinoma where 130 patients were randomized to gemcitabine and pertuzumab or placebo. ORR was 13.8% in the gemcitabine/pertuzumab arm vs. 4.6% in the gemcitabine/placebo arm. There was no statistically significant difference between PFS and OS in the two arms. The PFS and OS were 2.9 and 13.0 months in the gemcitabine/pertuzumab arm, respectively, and 2.6 and 13.1 months in the gemcitabine/placebo arm. (P=0.07 for PFS and P=0.65 for OS). In a biomarker analysis, those patients receiving pertuzumab who demonstrated low levels of HER3 mRNA had a lower risk of progression and trend for reduced risk of death, suggesting that low HER3 mRNA might be both a predictive and prognostic marker (33). Pertuzumab was also added to carboplatin-based chemotherapy in patients with platinum-sensitive, relapsed disease. A total of 149 patients received carboplatin and paclitaxel or gemcitabine with or without pertuzumab. There was no significant difference in PFS: 34.1 months for the combination vs. 37.3 months in the chemotherapy only arm (HR =1.16; 80% CI, 0.90-1.49; P=0.4487). The median OS for the combination was 28.2 months and not reached in the chemotherapy-only arm. The HR for death was 1.02 (P=0.9262) and confirmed that there was no difference between the two arms. In contrast to the prior trial, there was no treatment effect of pertuzumab on patients with low HER3 mRNA (34).
Finally, there have been several studies with lapatinib. Lapatinib was combined with carboplatin and paclitaxel in a phase I/II study in patients with stage III or IV relapsed ovarian cancer with an ORR of 50% in 21 patients (35). Single agent lapatinib was studied in the GOG biologic queue in persistent or recurrent disease and failed to show any objective responses in 25 patients with a median PFS of 1.8 months (36). In vitro, lapatinib enhances the efficacy of topotecan and so the combination of the two was explored in a phase I trial of 37 patients with solid tumors, including ovarian cancer. SD was seen in 18 patients (38). A phase II trial in 39 patients of the combination with recurrent ovarian cancer after first line chemotherapy only had a 14% PR rate (37).
Identifying mechanisms of resistance and future directions
Given disappointing clinical trial results with anti-EGFR and HER2 agents, greater insights into elucidating the mechanisms of resistance to these therapies and how they interact with other pathways are clearly needed. Unfortunately, such studies have been quite limited, which makes future pursuit of these pathways difficult. EGFR and HER2 may not be oncogenic drivers in ovarian cancer, as they are in other solid tumors. This notion is supported by the clinical trials described above which have not confirmed EGFR and HER2 as predictive markers. This makes it difficult to extrapolate the known mechanisms of resistance from other tumor types to ovarian cancer.
Nevertheless, some mechanisms of resistance have been identified in ovarian cancer and have mostly focused on EGFR. One of the most common resistance mechanisms, which holds true in other tumor types as well, is the activation of downstream signaling pathways, which can often make the inhibition of a solitary signal transduction pathway ineffective. Stimulation of EGFR in the cell membrane leads to the activation of two different but interconnected, pivotal pathways: the MAPK/extracellular signal regulated (MAPK/ERK) pathway and the PI3K/AKT/mTOR pathway (6,39). These pathways drive cell proliferation, survival and dissemination (40).
MAPK/ERK pathway
MAPK/ERK pathway activation and subsequent interactions are highly regulated events that become deregulated in cancer cells. The pathway begins with the activation of Ras, which initiates a multistep phosphorylation cascade that leads to the activation of MAPKs, ERK1, and ERK2 which ultimately regulate the transcription of molecules that are involved in cell proliferation (41). Patients with ovarian cancer frequently present with activation of the MAPK/ERK pathway due to activating mutations of KRAS or BRAF, which occur early in malignant transformation. KRAS and BRAF mutations are found in 27-36% and 33-50% of low grade serous ovarian carcinomas (LGSOC), respectively. KRAS mutations are found in 0-12% of high grade serous ovarian carcinomas (HGSOC) but BRAF mutations have not been described in HGSOC (40). These mutations can dysregulate kinase activity and hyperactivate the MAPK pathway during the induction and progression of tumorigenesis (42). The identification of these mutations has led to clinical trials with targeted BRAF and MEK inhibitor therapy in ovarian cancer (40). However, this pathway has not yet been evaluated in combination with anti-EGFR or HER2 therapy in ovarian cancer; however, it is still important to note that patients who harbor these mutations, resulting in pathway activation, may be immune to anti-EGFR and HER2 therapy as the downstream pathways are constitutively active. Further research of this pathway as a marker of resistance to anti EGFR and HER2 therapy is needed to confirm this hypothesis.
PI3K/AKT/mTOR pathway
The PI3K/AKT/mTOR pathway is involved in cellular motility, proliferation, differentiation, survival and tumorigenesis. It is activated in approximately 70% of ovarian cancers, leading to hyperactive signaling cascades. Some of the most common mechanisms that activate this pathway are mutations or amplification of PIK3CA, the catalytic subunit for PI3K, and loss of phosphatase and tensin homolog (PTEN), a tumor suppressor gene that normally regulates cell growth, survival, proliferation and angiogenesis through inhibition of the PI3K/AKT/mTOR pathway. Somatic changes in PIK3CA are frequently observed in ovarian cancer with 30.5% of cases having either alteration. PTEN loss has been reported in approximately 68% of ovarian cancer (43,44).
Given the limited activity of single agent EGFR TKI inhibition and the known activity of the PI3K/AKT/mTOR pathway in ovarian cancer, Glaysher et al. evaluated the effect of dual inhibition of EGFR and PI3K/mTOR on primary cell cultures from human ovarian tumors. The agents tested were TKIs erlotinib and gefitinib, ZSTK474, a PI3K inhibitor, and sirolimus, an mTOR inhibitor. All were initially tested as single agents and the majority of ovarian tumors were resistant to the EGFR inhibitors. The greatest single agent activity was seen with ZSTK474, suggesting the importance of PI3K signaling in these tumors. There was minimal response to sirolimus, which was not surprising given mTOR inhibitors predominantly elicit a cytostatic, rather than cytotoxic, response (39). However, when the agents were combined, there was greater synergistic activity with the combination of EGFR inhibitors with the PI3K and mTOR inhibitors; the most effective combination was an EGFR inhibitor and a PI3K inhibitor. Muranen et al. performed a study where ovarian tumor cells were treated with BEZ235, a PI3K/mTOR inhibitor, which decreased phosphorylation of proteins downstream of PI3K and mTOR and reduced cell proliferation markers. However, treatment with BEZ235 also induced upregulation and/or activation of multiple prosurvival proteins: cytoplasmic kinases, antiapoptotic proteins, transcription factors and several receptor tyrosine kinases, including EGFR and HER2. In a subsequent experiment, treatment with BEZ235 and EGFR inhibitors, PD168393 or gefitinib, resulted in marked cell death, suggesting the synergy of these two pathways (45).
Similar cross-talk has been demonstrated between EGFR and the Janus kinase (KAL)/STAT pathway that mediates the epithelial-mesenchymal transition and enhances migration (46). Furthermore, the endothelin-1 (ET-1) and the selective endothelin-A-receptor (ETAR), a G protein coupled receptor, are overexpressed in ovarian carcinomas. The autocrine ET-1/ETAR axis triggers several signaling pathways, which are involved in cell proliferation, survival, angiogenesis and invasion. ET-1 can transactivate EGFR through a Src-dependent mechanism. In vitro data showed that ET-1 induced rapid Src and EGFR phosphorylation and caused an increase in activation of MAPK and AKT in HEY cells, an ovarian cancer cell line. Treatment of HEY cells with gefitinib reduced ET-1 induced Src and EGFR activation; however, ET-1 mediated MAPK and AKT activation was incompletely reduced. ZD4054, an endothelin receptor antagonist, was then combined with gefitinib and resulted in greater inhibition of all of these pathways, again suggesting the importance of dual targeting (47).
The data described above clearly is in favor of dual targeting of pathways. Although this is all preclinical data so far, ideally, these combinations of anti-EGFR or HER2 therapies and downstream pathway inhibitors will be tested in clinical trials. This has already been done in breast cancer with the publication of the BOLERO-3 trial, which demonstrated the benefit of adding everolimus, an mTOR inhibitor, to trastuzumab and vinorelbine in patients with trastuzumab-resistant HER2 positive advanced breast cancer (48). One note of caution is that the combination of targeted therapies can result in increased toxicity, which must be balanced against quality of life in patients with advanced disease.
Other mechanisms of resistance
Another proposed mechanism has been resistance to autophagic cell death upon increased EGFR expression due to stabilization of the facilitated glucose transporter sodium/glucose cotransporter 1 (SGLT1) (6). SGLT1 allows cancer cells to uptake glucose, regardless of the level of extracellular glucose, for their survival (49). The cells are able to uptake enough glucose for ATP generation, via glycolysis, which prevents them from dying. As such, the presence of EGFR maintains the basal intracellular glucose levels and cells do not undergo autophagic death. Thus, even in the presence of anti EGFR directed agents, EGFR may provide tumor cells with an increased survival capacity. It is hypothesized that inhibition of this function in combination with anti-EGFR directed agents might be required to overcome resistance (49).
In preclinical models, EGFR has also been shown to induce platelet-activating factor (PAF) production, which is a pro-inflammatory lipid mediator that binds to the PAF-receptor (PAFR) and plays a significant role in oncogenic transformation, tumor growth, neoangiogenesis, and metastasis in ovarian cancer. Yu et al. demonstrated that the epidermal growth factor increases PAF production in CAOV3 and SKOV3 ovarian cancer cell lines. Although inhibition of EGFR and/or PAFR blocks PAF production, crosstalk can occur bi-directionally between EGFR and PAFR and epidermal growth factor induced PAF production may result in a positive feedback mechanism that acts on the PAFR to promote ovarian cancer progression. Dual inhibition of EGFR and PAFR may be one way to overcome resistance to anti EGFR directed therapies (50).
Conclusions
EGFR and HER2 expression in ovarian cancer is quite variable. These targets have been extensively studied with discouraging results and at this point, there seems to be little role for anti-EGFR or HER2 directed therapies in ovarian cancer outside of clinical trials. Studying resistance mechanisms may help identify why these targets have not been successful and more ideally, there will be more research into this area. Current data stresses the importance of dual targeting with anti-EGFR or HER2 and downstream pathway inhibitors. There has been some recent work in uterine carcinoma demonstrating the preclinical efficacy of neratinib, a small TKI against EGFR and HER2 (51), and taselisib, a selective inhibitor of PIK3CA, on PIK3CA-mutated and HER2 amplified in uterine serous carcinoma cell lines and mouse xenografts (52). Hopefully, these drugs will be evaluated in ovarian cancer as well in the future. There are still possibilities to ‘salvage’ EGFR and HER2 targeting in ovarian cancer, gain greater insight on how these pathways contribute to tumorigenesis in this disease and identify a clinical benefit.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting”. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.01.01). The series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” was commissioned by the editorial office without any funding or sponsorship. FM and ET served as the unpaid Guest Editors of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer 2009;9:167-81. [PubMed]
- Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer 2013;13:663-73. [PubMed]
- Reyes HD, Thiel KW, Carlson MJ, et al. Comprehensive profiling of EGFR/HER receptors for personalized treatment of gynecologic cancers. Mol Diagn Ther 2014;18:137-51. [PubMed]
- Press MF, Lenz HJ. EGFR, HER2 and VEGF pathways: validated targets for cancer treatment. Drugs 2007;67:2045-75. [PubMed]
- Teplinsky E, Muggia F. Targeting HER2 in ovarian and uterine cancers: Challenges and future directions. Gynecol Oncol 2014;135:364-70. [PubMed]
- Hirte HW. Profile of erlotinib and its potential in the treatment of advanced ovarian carcinoma. Onco Targets Ther 2013;6:427-35. [PubMed]
- Lafky JM, Wilken JA, Baron AT, et al. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta 2008;1785:232-65.
- Skirnisdóttir I, Sorbe B, Seidal T. The growth factor receptors HER-2/neu and EGFR, their relationship, and their effects on the prognosis in early stage (FIGO I-II) epithelial ovarian carcinoma. Int J Gynecol Cancer 2001;11:119-29. [PubMed]
- Tas F, Karabulut S, Serilmez M, et al. Increased serum level of epidermal growth factor receptor (EGFR) is associated with poor progression-free survival in patients with epithelial ovarian cancer. Cancer Chemother Pharmacol 2014;73:631-7. [PubMed]
- Gordon AN, Finkler N, Edwards RP, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer 2005;15:785-92. [PubMed]
- Hirte H, Oza A, Swenerton K, et al. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND.149). Gynecol Oncol 2010;118:308-12. [PubMed]
- Blank SV, Christos P, Curtin JP, et al. Erlotinib added to carboplatin and paclitaxel as first-line treatment of ovarian cancer: a phase II study based on surgical reassessment. Gynecol Oncol 2010;119:451-6. [PubMed]
- Warner E, Liebes L, Levinson B, et al. Continuous-infusion topotecan and erlotinib: a study in topotecan-pretreated ovarian cancer assessing shed collagen epitopes as a marker of invasiveness. Oncologist 2014;19:250. [PubMed]
- Vergote IB, Jimeno A, Joly F, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J Clin Oncol 2014;32:320-6. [PubMed]
- Schilder RJ, Sill MW, Chen X, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res 2005;11:5539-48. [PubMed]
- Campos S, Hamid O, Seiden MV, et al. Multicenter, randomized phase II trial of oral CI-1033 for previously treated advanced ovarian cancer. J Clin Oncol 2005;23:5597-604. [PubMed]
- Secord AA, Blessing JA, Armstrong DK, et al. Phase II trial of cetuximab and carboplatin in relapsed platinum-sensitive ovarian cancer and evaluation of epidermal growth factor receptor expression: a Gynecologic Oncology Group study. Gynecol Oncol 2008;108:493-9. [PubMed]
- Konner J, Schilder RJ, DeRosa FA, et al. A phase II study of cetuximab/paclitaxel/carboplatin for the initial treatment of advanced-stage ovarian, primary peritoneal, or fallopian tube cancer. Gynecol Oncol 2008;110:140-5. [PubMed]
- Schilder RJ, Pathak HB, Lokshin AE, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol Oncol 2009;113:21-7. [PubMed]
- Seiden MV, Burris HA, Matulonis U, et al. A phase II trial of EMD72000 (matuzumab), a humanized anti-EGFR monoclonal antibody, in patients with platinum-resistant ovarian and primary peritoneal malignancies. Gynecol Oncol 2007;104:727-31. [PubMed]
- Reibenwein J, Krainer M. Targeting signaling pathways in ovarian cancer. Expert Opin Ther Targets 2008;12:353-65. [PubMed]
- Serrano-Olvera A, Dueñas-González A, Gallardo-Rincón D, et al. Prognostic, predictive and therapeutic implications of HER2 in invasive epithelial ovarian cancer. Cancer Treat Rev 2006;32:180-90. [PubMed]
- Raspollini MR, Amunni G, Villanucci A, et al. HER-2/neu and bcl-2 in ovarian carcinoma: clinicopathologic, immunohistochemical, and molecular study in patients with shorter and longer survival. Appl Immunohistochem Mol Morphol 2006;14:181-6. [PubMed]
- Tuefferd M, Couturier J, Penault-Llorca F, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS One 2007;2:e1138 [PubMed]
- Lassus H, Leminen A, Vayrynen A, et al. ERBB2 amplification is superior to protein expression status in predicting patient outcome in serous ovarian carcinoma. Gynecol Oncol 2004;92:31-9. [PubMed]
- Verri E, Guglielmini P, Puntoni M, et al. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: evaluation of its prevalence and prognostic significance. Clinical study. Oncology 2005;68:154-61. [PubMed]
- Nielsen JS, Jakobsen E, Hølund B, et al. Prognostic significance of p53, Her-2, and EGFR overexpression in borderline and epithelial ovarian cancer. Int J Gynecol Cancer 2004;14:1086-96. [PubMed]
- Zhao D, Zhang F, Zhang W, et al. Prognostic role of hormone receptors in ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer 2013;23:25-33. [PubMed]
- Jelovac D, Emens LA. HER2-directed therapy for metastatic breast cancer. Oncology (Williston Park) 2013;27:166-75. [PubMed]
- Jhaveri K, Esteva FJ. Pertuzumab in the treatment of HER2+ breast cancer. J Natl Compr Canc Netw 2014;12:591-8. [PubMed]
- Bookman MA, Darcy KM, Clarke-Pearson D, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol 2003;21:283-90. [PubMed]
- Gordon MS, Matei D, Aghajanian C, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. J Clin Oncol 2006;24:4324-32. [PubMed]
- Makhija S, Amler LC, Glenn D, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol 2010;28:1215-23. [PubMed]
- Kaye SB, Poole CJ, Dańska-Bidzińska A, et al. A randomized phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. Ann Oncol 2013;24:145-52. [PubMed]
- Rivkin SE, Muller C, Iriarte D, et al. A phase I/II lapatinib plus carboplatin and paclitaxel in stage III or IV relapsed ovarian cancer patients. J Clin Oncol 2008;26:abstr 5556.
- Garcia AA, Sill MW, Lankes HA, et al. A phase II evaluation of lapatinib in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. Gynecol Oncol 2012;124:569-74. [PubMed]
- Lheureux S, Krieger S, Weber B, et al. Expected benefits of topotecan combined with lapatinib in recurrent ovarian cancer according to biological profile: a phase 2 trial. Int J Gynecol Cancer 2012;22:1483-8. [PubMed]
- Molina JR, Kaufmann SH, Reid JM, et al. Evaluation of lapatinib and topotecan combination therapy: tissue culture, murine xenograft, and phase I clinical trial data. Clin Cancer Res 2008;14:7900-8. [PubMed]
- Glaysher S, Bolton LM, Johnson P, et al. Targeting EGFR and PI3K pathways in ovarian cancer. Br J Cancer 2013;109:1786-94. [PubMed]
- Burotto M, Chiou VL, Lee JM, et al. The MAPK pathway across different malignancies: a new perspective. Cancer 2014;120:3446-56. [PubMed]
- Scartozzi M, Bearzi I, Berardi R, et al. Epidermal growth factor receptor (EGFR) downstream signalling pathway in primary colorectal tumours and related metastatic sites: optimising EGFR-targeted treatment options. Br J Cancer 2007;97:92-7. [PubMed]
- Pohl G, Ho CL, Kurman RJ, et al. Inactivation of the mitogen-activated protein kinase pathway as a potential target-based therapy in ovarian serous tumors with KRAS or BRAF mutations. Cancer Res 2005;65:1994-2000. [PubMed]
- Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch Gynecol Obstet 2014;290:1067-78. [PubMed]
- Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 2009;9:415-28. [PubMed]
- Muranen T, Selfors LM, Worster DT, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 2012;21:227-39. [PubMed]
- Colomiere M, Ward AC, Riley C, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer 2009;100:134-44. [PubMed]
- Rosanò L, Cianfrocca R, Masi S, et al. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci U S A 2009;106:2806-11. [PubMed]
- André F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:580-91. [PubMed]
- Weihua Z, Tsan R, Huang WC, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 2008;13:385-93. [PubMed]
- Yu Y, Zhang X, Hong S, et al. Epidermal growth factor induces platelet-activating factor production through receptors transactivation and cytosolic phospholipase A2 in ovarian cancer cells. J Ovarian Res 2014;7:39. [PubMed]
- Schwab CL, English DP, Roque DM, et al. Neratinib shows efficacy in the treatment of HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol 2014;135:142-8. [PubMed]
- Lopez S, Schwab CL, Cocco E, et al. Taselisib, a selective inhibitor of PIK3CA, is highly effective on PIK3CA-mutated and HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol 2014;135:312-7. [PubMed]


