
Intraoperative radiotherapy in the treatment of gastrointestinal malignancies
The treatment of gastrointestinal malignancies with external beam radiation therapy (EBRT) is limited by the normal tissue tolerance of various abdominal and pelvic organs including stomach, bowel, liver and kidneys. Intraoperative radiotherapy (IORT) has emerged as a technique to deliver a single high dose of radiation to a target volume at the time of an operation. IORT is typically performed in combination with EBRT, chemotherapy and surgical resection. The advantage of this technique is the delivery of a biologically potent dose of radiation with rapid dose fall off and the ability to move and shield nearby organs at risk for normal tissue complication.
Delivery of IORT in the abdomen and pelvis is achieved primarily by two techniques: intraoperative electron radiation therapy (IOERT) and high-dose rate brachytherapy (HDR-IORT). IOERT previously required transporting a patient from the operating room (OR) to the radiation oncology department or having an OR equipped with a linear accelerator. More recently, mobile units have been developed to increase the versatility of this therapy. The delivery of electrons to a discrete area necessitates the use of a rigid applicator or cone, a requirement that can be technically challenging depending on the target area and patient position. Multiple electron energies are available which allows the desired depth of treatment to be modified.
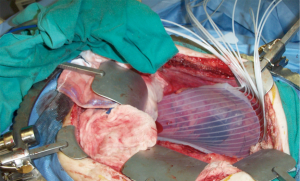
HDR-IORT uses an iridium-192 source housed in a remote afterloading device. The iridium source has a 74-day half-life and must be replaced throughout the year. High energy photons (370 keV) are emitted, thus facilities using this technique require appropriate shielding. HDR-IORT is accomplished by the placement of a flexible applicator with multiple channels in the operative bed (Figure 1). The radioactive source passes from the remote afterloading device through catheters into the channels of the applicator at pre-determined positions and dwell times. The short range of these photons limits this technique primarily to superficial/microscopic disease only (0.5 cm thickness). In both IOERT and HDR-IORT, close consultation and collaboration with the surgeon and pathologist are critical in defining the target volume.
The feasibility, volume and IORT dose given depend on normal tissue tolerance, initially derived from canine models at the National Cancer Institute (NCI) and Colorado State University. These studies have demonstrated that gastrointestinal mucosa is particularly radiosensitive, and IORT doses of 15-20 Gy can result in strictures, obstructions, ulceration or perforations (1). While many organs can be mobilized away from the treatment area and/or shielded, this is not feasible for deep nerves in the lumbar and sacral region where IORT may result in peripheral neuropathy. A trial by the NCI randomized patients with resectable retroperitoneal sarcomas to 50-55 Gy postoperative EBRT alone or 20 Gy IOERT followed by 35-40 Gy EBRT. Radiation-related peripheral neuropathy was seen in 9 of 15 patients in the IOERT arm (2). Peripheral nerves are therefore a dose limiting structure, with a maximum tolerated dose of approximately 15 Gy based on the previously mentioned studies (3,4).
IORT has been used in the treatment of numerous gastrointestinal malignancies, primarily in disease sites with high rates of local failure. The majority of published experience has been in stomach, pancreatic and rectal cancers.
Gastric cancer
In a landmark study, Gunderson et al. published the patterns of failure in patients with gastric cancer following resection based on second look surgeries. Locoregional failure occurred in 67% of patients, 54% of which were in the gastric bed and 26% in the anastomosis or stump (5,6). Japanese investigators sought to evaluate whether the addition of IORT to surgery improved outcomes. Patients were randomized to surgery alone or surgery with 28-35 Gy IORT. A long-term survival benefit with IORT was seen in a subgroup of patients with advanced disease (7). At the NCI, a small randomized study compared gastrectomy and 20 Gy IORT to the gastric bed to gastrectomy (either alone or followed by conventional postoperative EBRT) for advanced-stage lesions. Locoregional control was significantly improved in the patients who were treated with IORT (8). No randomized comparisons of IORT exist with adjuvant chemoradiation therapy as the control arm which is considered standard of care in advanced gastric cancer (9). A retrospective series from China compared outcomes of patients receiving 12-15 Gy IOERT followed by chemoradiotherapy to adjuvant chemoradiotherapy alone. IORT was well tolerated and associated with improvements in locoregional control and disease-free survival (10).
Pancreatic cancer
Pancreatic cancers are characterized by high rates of both local and distant failure. In patients amenable to a potentially curative pancreaticoduodenectomy, rates of positive retroperitoneal margins are as high as 40% (11). IORT has been investigated as an adjuvant therapy, with most reports limited to single institution series. The available data suggest there is a consistent local control benefit with IORT, while the effect on survival has been variable (12-14). With recent improvements in chemotherapy and increasing utilization of neoadjuvant chemoradiotherapy, the role of IORT in potentially resectable pancreatic cancers is not clear at present. In patients with locally advanced pancreatic cancer, local failure occurs in the majority of patients despite EBRT and chemotherapy (15). In part, this may relate to the inadequate doses administered to control gross disease. Dose escalation with IORT has been investigated as a means of improving local control and overall outcomes. A report from the Mayo Clinic investigating the addition of IORT to EBRT demonstrated an increase in local control but no improvement in survival (16). The Radiation Therapy Oncology Group (RTOG) prospectively evaluated combination 20 Gy IORT followed by chemoradiotherapy to 50.4 Gy. The results were disappointing with a median survival similar to conventional treatment (17). Multiple single institution series have been published demonstrating high rates of local control but no consistent improvement in overall survival. In patients experiencing pain related to their primary disease, IORT has been shown to effectively palliate this symptom (18).
Rectal cancer
An area of active investigation with IORT is in the treatment of recurrent or primary locally advanced rectal cancers. Locally advanced rectal cancers typically involve fixation or adherence to adjacent structures such as the sacrum or pelvic side wall. With no upfront therapy, these tumors are considered unresectable or have a high probability of gross/microscopic residual disease following resection. Even after preoperative EBRT and subsequent complete resection, these tumors have a high probability of recurrence. IORT has been utilized in this circumstance to improve local control. Investigators at Massachusetts General Hospital (MGH) reported their experience with IOERT following preoperative EBRT for locally advanced rectal cancer. Patients at higher risk for recurrence (gross residual tumor, positive or close margins) were selected for IORT at doses ranging from 10-20 Gy depending on the extent of residual disease. The local control rate in the IORT group compared favorably to the lower risk group not receiving additional radiation therapy (19). The complication rate for those treated with IOERT was 15%. With a similar treatment approach, investigators at the Mayo Clinic also reported high rates of local control in this unfavorable patient population (20).
IORT may also be utilized in the management of recurrent rectal cancers, most often using a multimodality approach. Experience from the Mayo Clinic suggests IOERT and EBRT following palliative surgical resection improves local control and survival outcomes compared to resection alone in locally recurrent rectal cancers (21). The likelihood of achieving margin negative resection is considerably less in this population, thus neoadjuvant chemoradiation prior to surgery should be pursued to increase the rate of R0 resection (22). Despite this, R0 rates, local control and long-term survival are much lower compared to treatment in the primary setting.
Conclusions
IORT appears to be a useful technique in the combined modality management of locally advanced or recurrent gastrointestinal malignancies. Enhancement of the therapeutic ratio is achieved by the ability to exclude sensitive normal tissue from the operative bed. When conducted carefully, there are consistently improved rates of local control with low toxicity rates across several disease sites. Overall survival improvements are variable which relates to the significant competing risk of distant failure in many of these malignancies. Improvements in systemic therapy may enhance the relative benefit of local control. The increasing mobility of modern IORT units is likely to increase utilization of this technique. Prospective evaluation in the modern era will be necessary to determine efficacy, particularly as competing technology for dose escalation such as intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) become more prevalent.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frederik Wenz and Elena Sperk) for the series “Intraoperative Radiotherapy” published in Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.04.05). The series “Intraoperative Radiotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sindelar WF, Kinsella TJ. Normal tissue tolerance to intraoperative radiotherapy. Surg Oncol Clin N Am 2003;12:925-42. [PubMed]
- Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg 1993;128:402-10. [PubMed]
- LeCouteur RA, Gillette EL, Powers BE, et al. Peripheral neuropathies following experimental intraoperative radiation therapy (IORT). Int J Radiat Oncol Biol Phys 1989;17:583-90. [PubMed]
- Kinsella TJ, DeLuca AM, Barnes M, et al. Threshold dose for peripheral neuropathy following intraoperative radiotherapy (IORT) in a large animal model. Int J Radiat Oncol Biol Phys 1991;20:697-701. [PubMed]
- Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1982;8:1-11. [PubMed]
- Gunderson LL. Gastric cancer--patterns of relapse after surgical resection. Semin Radiat Oncol 2002;12:150-61. [PubMed]
- Abe M, Takahashi M, Ono K, et al. Japan gastric trials in intraoperative radiation therapy. Int J Radiat Oncol Biol Phys 1988;15:1431-3. [PubMed]
- Sindelar WF, Kinsella TJ, Tepper JE, et al. Randomized trial of intraoperative radiotherapy in carcinoma of the stomach. Am J Surg 1993;165:178-86; discussion 186-7. [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [PubMed]
- Zhang Q, Tey J, Peng L, et al. Adjuvant chemoradiotherapy with or without intraoperative radiotherapy for the treatment of resectable locally advanced gastric adenocarcinoma. Radiother Oncol 2012;102:51-5. [PubMed]
- Willett CG, Lewandrowski K, Warshaw AL, et al. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993;217:144-8. [PubMed]
- Zerbi A, Fossati V, Parolini D, et al. Intraoperative radiation therapy adjuvant to resection in the treatment of pancreatic cancer. Cancer 1994;73:2930-5. [PubMed]
- Reni M, Panucci MG, Ferreri AJ, et al. Effect on local control and survival of electron beam intraoperative irradiation for resectable pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2001;50:651-8. [PubMed]
- Ogawa K, Karasawa K, Ito Y, et al. Intraoperative radiotherapy for resected pancreatic cancer: a multi-institutional retrospective analysis of 210 patients. Int J Radiat Oncol Biol Phys 2010;77:734-42. [PubMed]
- . Radiation therapy combined with Adriamycin or 5-fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Gastrointestinal Tumor Study Group. Cancer 1985;56:2563-8. [PubMed]
- Roldan GE, Gunderson LL, Nagorney DM, et al. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer 1988;61:1110-6. [PubMed]
- Tepper JE, Noyes D, Krall JM, et al. Intraoperative radiation therapy of pancreatic carcinoma: a report of RTOG-8505. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1991;21:1145-9. [PubMed]
- Fossati V, Cattaneo GM, Zerbi A, et al. The role of intraoperative therapy by electron beam and combination of adjuvant chemotherapy and external radiotherapy in carcinoma of the pancreas. Tumori 1995;81:23-31. [PubMed]
- Nakfoor BM, Willett CG, Shellito PC, et al. The impact of 5-fluorouracil and intraoperative electron beam radiation therapy on the outcome of patients with locally advanced primary rectal and rectosigmoid cancer. Ann Surg 1998;228:194-200. [PubMed]
- Mathis KL, Nelson H, Pemberton JH, et al. Unresectable colorectal cancer can be cured with multimodality therapy. Ann Surg 2008;248:592-8. [PubMed]
- Suzuki K, Gunderson LL, Devine RM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer 1995;75:939-52. [PubMed]
- Roeder F, Goetz JM, Habl G, et al. Intraoperative Electron Radiation Therapy (IOERT) in the management of locally recurrent rectal cancer. BMC Cancer 2012;12:592. [PubMed]


