
The management of brain necrosis as a result of SRS treatment for intra-cranial tumors
Introduction
Brain necrosis resulting from therapeutic irradiation to the whole brain, partial brain or stereotactic radiosurgery (SRS) is commonly referred to as radionecrosis (RN). RN is an infrequent yet well recognized SRS treatment risk for malignant, metastatic and certain benign tumors such as AVM’s. The necrosis results from avascularization of the tissue at the site of the SRS target. The incidence of RN from SRS has been reported to occur in as many as 50% of treated metastatic lesions (1-6). Fortunately, most necrotic sites remain asymptomatic and heal with time over weeks to months. Factors which influence necrosis mainly include target dose and volume (7,8). Reducing either the dose or volume treated in order to avoid the RN risk has to be weighed against the importance of achieving high levels of tumor over the patient expected life time. The symptoms of RN depend on the location and function of the brain at the injury site. These symptoms can range from headaches, fatigue, nausea, imbalance, extremity weakness/numbness, speech deficits, and seizures to a combination of the above.
Minniti et al. reported on the risk of SRS induced brain necrosis observed in 206 patients with 310 cerebral metastases (9). RN was radiographically documented in 24% of treated lesions with 10% of patients having new neurologic symptoms and 14% remaining asymptomatic. Median time to symptomatic necrosis was 11 months (range, 2-32 months). In their study, the development of RN was determined by MRI imaging. Volume and dose were independent risk factors for necrosis. The risk of necrosis was greater than 10% when the brain volume receiving the 12 Gy in a single fraction was greater than 8.5 cm3.
In our practice we have found that tumor growth versus SRS induced necrosis can be difficult to distinguish on a contrast enhanced brain MRI alone. Needless to say, the differentiation is important to recognize in an effort to provide appropriate patient management if new or worsening neurologic symptoms are present. If incorrectly diagnosed for tumor progression, SRS or whole brain irradiation retreatment could result in potentially larger areas of symptomatic necrosis. This article will review the current trends in identifying and managing patients that develops symptomatic SRS induced brain necrosis and conclude with a treatment algorithm guideline.
Radiographic differential for SRS brain necrosis
Despite the best efforts of the neuroradiology community, distinguishing SRS related brain necrosis from tumor recurrence and/or tumor progression as well as treatment related pseudo-response and pseudo-progression remains elusive (10-13). There is no single conclusive imaging methodology to distinguish between these processes. It is often the clinical course, a brain biopsy, or imaging over a lengthy follow-up interval that reveal recurrent tumor from radiation necrosis. The current imaging armamentarium available includes gadolinium enhanced MRI, MR Diffusion, MR Perfusion, MR Spectroscopy and PET-CT. Interpretation of these studies is aided by patient symptoms, tumor type and grade and treatment history including time course as well as the radiation dose and volume.
RN tends to occur at site of maximum radiation dose in the region of the tumor bed. Radiation disrupts the blood brain barrier (BBB) and its effect on blood vessels (occlusive vasculopathy) can potentiate ischemia (14,15). Analysis is further complicated by the fact that RN and viable tumor can coexist as a dynamic process and, although it is not always irreversible, may spontaneously regress and completely resolve. Also, anti-angiogenic (VEGF) drugs, such as bevacizumab, can normalize BBB permeability resulting in improvement of both tumors as well as RN.
MRI
Enhancement in a treated tumor bed occurs as a result of disruption of the BBB. This can be as a result of tumor recurrence other causes include post-surgical granulation tissue, ischemia, infection and RN. By conventional MR imaging it can be impossible to distinguish between recurrent tumor and RN. Features pointing to RN include: (I) development of lesion away from primary tumor site; (II) development of multiple lesions; (III) lesion developing in the periventricular region as this area is prone to ischemia being in the end arteriole territory; (IV) lesion developing in a zone of atrophy and (V) lesion developing in the contralateral hemisphere. These features are useful in the case of a primary brain neoplasm but not so useful in metastatic disease (16).
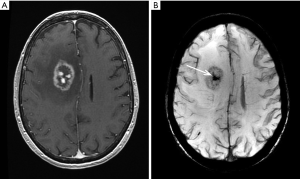
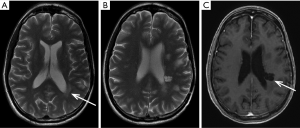
RN may be suspected if: (I) an enhancing lesion develops in the bed of a previously non enhancing tumor; (II) there is development of lesion in an area of radiation induced leucoencephalopathy; (III) there is relatively little mass effect for size of the lesion; (IV) the lesion is peripherally enhancing with central necrosis. The enhancement may have an eccentric leading edge sometimes described as a “wavefront” (Figure 1A); (V) there is nodular enhancement in the zone of necrosis sometimes described as a “Swiss Cheese” enhancement (Figure 1A); (VI) there is associated hemosiderin and calcifications (Figure 1B); (VII) involvement of the corpus callosum and (VIII) dilatation of adjacent perivascular spaces is present (Figure 2) (17). Also see example in Figure 2A-C.
MR diffusion
Diffusion-weighted imaging (DWI) is based on the detection of a change in the random or Brownian motion of protons in cellular and interstitial water. When the diffusion of water molecules is diminished this is called “restricted” and when increased it is called “facilitated”. This “diffusability” can be measured on apparent diffusion coefficient (ADC) maps and enables characterization of disease processes. Acute infarct is typically characterized by restricted diffusion. Certain tumors such as epidermoids demonstrate marked restricted diffusion and this feature is vital in making the diagnosis. Tumors that are highly cellular such as lymphoma and meningioma can also have restricted diffusion.
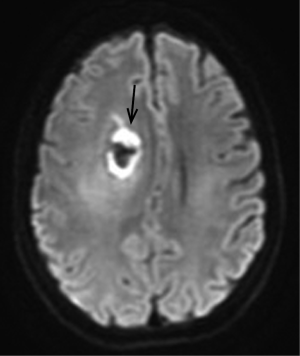
There is overlap in the DWI appearance of RN and recurrent tumor. Occlusive vasculopathy as a result of radiation can result in ischemic change, with restricted diffusion and low ADC values (Figure 3). In the chronic stage there is a trend towards higher ADC values (facilitated diffusion) with RN (18). Other investigators using diffusion tensor imaging (DTI) have demonstrated the opposite higher ADC values in recurrent tumor as opposed to cases of RN (19).
MR perfusion
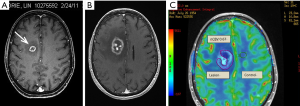
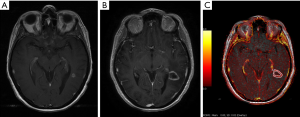
Dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging can estimate tissue microvascular density by measuring relative cerebral blood volume (rCBV). Radiation necrosis typically results in endothelial cell damage and small-vessel injury, resulting in decreased tissue perfusion. In contrast, tumor recurrence promotes angiogenesis and microvascular proliferation, helping to sustain tumor growth. Measurements are obtained in the region of the putative area of necrosis/recurrent tumor and compared to normal tissue in the contralateral hemisphere resulting in a ratio (rCBV). rCBV >1.5 is indicative of recurrent tumor, rCBV <0.7 is indicative of RN (Figure 4A-C). Dynamic contrast enhanced (DCE) imaging with calculation of tissue permeability (K trans) provides similar results but with the added advantage of diminished susceptibility artefact and higher spatial resolution (20-22) (Figure 5A-C).
MR spectroscopy
The resonant frequency of protons is altered by their chemical environment. This forms the basis for MR spectroscopy. The spectra typically displayed are expressed in units of parts per million (ppm). Commonly encountered metabolites in normal brain tissue include N-acetyl aspartate (NAA), choline and creatine. Lactate and lipids can also be detected.
NAA (2.0 ppm) is the largest peak in normal spectra. It is considered a marker of normal neuronal function. A decrease in NAA indicates axonal injury or neuronal loss. Choline is a component of the cell membrane. An increase in the choline peak (3.2 ppm) reflects an increase in membrane synthesis and increased cellular turnover/prolferation. Creatine (3.0 ppm) serves as a marker of the energy reserves in the cell and remains relatively stable even when disease is encountered. The lactate peak (1.32 ppm) consists of two distinct resonant peaks (a doublet), its presence indicates anaerobic metabolism (23).
Cho/NAA and Cho/Cr ratios tend to be significantly higher in recurrent tumor than in RN, whereas the NAA/Cr ratios are lower in recurrent tumor than in radiation injury (Figure 6A-C). Lipids are seen with necrosis and can be seen in tumors. Radiation sensitivity, specificity, and diagnostic accuracy of 3D 1H-MR spectroscopy for diagnosing recurrent tumor were 94.1%, 100%, and 96.2%, respectively, based on the cutoff values of 1.71 for Cho/Cr or 1.71 for Cho/NAA (23).

PET CT
Glucose metabolism can be measured by the uptake of [18F] FDG. Uptake is measured relative to cortex. Tumor cells generally have high rates of glucose metabolism, and therefor demonstrate high FDG uptake. Conversely, RN will have lower glucose metabolism and therefore low FDG uptake (Figure 7A,B). Low grade gliomas may have lower metabolism when compared to cortex. FDG PET may be less sensitive in differentiating recurrence from radiation injury in these tumors. FDG PET can be false positive due to volume averaging with the cortex and seizure activity or false negative due to a mixture of a large percentage of necrosis with limited tumor. Imaging in the first three months following radiation or surgery may result in false-positive scans caused by increased metabolic activity arising from the healing process.
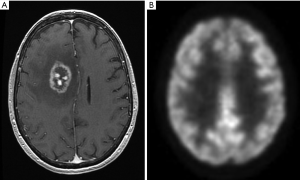
Evaluation is limited by low spatial resolution and beam hardening artefact from the skull base that makes it difficult to evaluate lesions in the temporal lobes, cerebellum and brain stem. In a study by Ricci et al., PET findings were confirmed histologically in 31 patients. With contralateral white matter as the standard of comparison, the PET scan sensitivity and specificity were found to be 86% and 22%, respectively. With contralateral gray matter as the reference standard, the sensitivity and specificity became 73% and 56%, respectively. Overall, nearly one third of the patients would have been treated inappropriately in either scheme had the PET scan been the sole determinant of therapy. They conclude that ability of FDG PET to differentiate recurrent tumor from radiation necrosis is limited (24).
Imaging preference at our medical center
After SRS treatment for brain metastases, patients are usually followed on an every 2-3 months bases with serial contrasted enhanced MRI scans which include perfusion and diffusion. If the patient’s clinical findings and MRI suggests probable symptomatic RN and treatment intervention other than steroids is warranted, spectroscopy or a PET CT will be obtained for corroboration of necrosis. Spectroscopy is preferred because of its sensitivity over PET CT but is seldom covered by insurance.
Corticosteroid use in the management of brain necrosis
For patients who present with radiographic changes on MRI and symptoms suggestive of necrosis, the mainstay of treatment is corticosteroids until the lesion heals or symptoms resolve. During this time, in order to avoid the long-term effect of corticosteroids, the dose should be the lowest possible to reduce the edema. Unfortunately, even with small doses, not all patients tolerate the sometimes immediate effects of corticosteroids which include anxiety, sleeplessness, aggressive behavior, depression, GI irritation, increased appetite and swelling of the hands, feet and face.
Bevacizumab in the management of SRS brain necrosis
Evidence has implicated the upregulation of VEGF in the pathogenesis of radiation induced brain necrosis making it an attractive therapeutic target (25,26). Bevacizumab, a humanized monoclonal antibody directed against vascular endothelial growth factor (VEGF), is an anti-angiogenic therapy with the best-supported evidence in patients with symptomatic RN who respond poorly to corticosteroids as initial symptomatic management (27-30). Pharmacologic blockage of VEGF with bevacizumab can restore BBB function and markedly reduce cerebral edema and mass effect with subsequent improvement in neurologic symptoms (27).
Gonzalez et al. retrospectively reviewed 8 patients with RN treated with bevacizumab and reported a mean reduction in T1-weighted post-gadolinium abnormalities of 48% and a reduction in the fluid-attenuated inversion-recovery (FLAIR) abnormalities of 60% (27). A significant reduction in steroid requirement by 8.6 mg daily was also observed. Subsequently, a double-blind, placebo-controlled trial conducted by Levin et al. evaluated 14 patients with radiographic or biopsy confirmed RN, randomized to receive bevacizumab 7.5 mg/kg or intravenous saline at 3-week intervals for four treatments (28). All bevacizumab treated patients (five of five randomized and seven of seven crossover), and none of the placebo-treated patients, demonstrated a decrease in FLAIR and T1 post-gadolinium abnormalities as well as improvement in neurologic symptoms. At median follow-up of ten months after four doses of bevacizumab, only two patients experienced a recurrence of radiographic changes consistent with progressive RN for which bevacizumab was resumed for one to two doses. This study demonstrated the efficacy of bevacizumab in the treatment of cerebral RN and was later confirmed in a retrospective review published by Sadraei et al. (29). Twenty-three of 24 patients with RN treated with varied dosing regimens of bevacizumab demonstrated radiographic improvement with mean reduction in T1-weighted post-gadolinium abnormality of 48.1% and average reduction in FLAIR abnormalities of 53.7%. They additionally reported a daily dose reduction of dexamethasone by 9.4 mg after the initiation of bevacizumab. Therapy was well tolerated with one grade three adverse event. Boothe et al. additionally retrospectively reviewed a series of 11 patients treated with bevacizumab for SRS induce brain necrosis (30). The mean percentage decrease in post-gadolinium and FLAIR volume was 64.4% and 64.3% respectively, with reduction in steroid requirements and all but one patient demonstrated improvement or stability of neurologic symptoms.
Questions remain regarding the ideal dosing and treatment duration for bevacizumab in RN, without an observed difference in clinical and radiographic outcomes. Bevacizumab is most commonly administered at 10 mg/kg every two weeks or 7.5 mg/kg every three weeks. Based on the only randomized, double-blind, placebo-controlled trial and retrospective analyses, it is reasonable to administer bevacizumab for approximately 4 doses followed by a period of observation with surveillance MRI every six to eight weeks. In the event of recurrent RN, this may be resumed for a short duration.
Bevacizumab to date is the best-supported therapy for radiation induced brain necrosis and should be considered in patients with progressive neurologic symptoms refractory to more conservative therapies. Bevacizumab offers symptomatic relief, reduction in steroid requirements and dramatic radiographic response and may obviate the need for surgery. Due to potential toxicities including hypertension (4-16%), proteinuria (0-3.2%), wound healing complications (0-2.4%) and vascular events such as intra-cranial hemorrhage (all grades/>grade 3, 0-3%/0%) and venous thromoboembolism (2.0-12.6%), careful selection of patients should be made prior to initiating treatment (31). Contra-indications to bevacizumab include patients with intracerebral hemorrhage and in patients with metastases at high risk for hemorrhage such as melanoma.
Surgical management of SRS brain necrosis
RN often presents as a mass producing lesion necessitating surgical removal. When corticosteroid and anti-angiogenic strategies are incapable of eradicating an edema-generating lesion causing mass effect in the setting of neurological deterioration, surgical removal must be considered. The neurosurgical approach to such lesions is no different than that of a primary tumor. The goal is to resect necrotic tumor and brain tissue that serves as an edema-generator. Standard neuronavigation and microsurgical techniques are utilized to resect enhancing areas of necrotic tumor and brain tissue. Under the operating microscope, areas of radiation necrosis often appear as leathery scar-like areas adjacent to edematous white matter. Careful resection of enhancing lesional tissue often provides significant reversal of the edema-producing effects of the areas of RN, and patients may be able to be weaned from corticosteroids. Many patients will have significant improvements in neurological function. Most operations to resect areas of RN in the brain result in similar outcomes as with a tumor resection in terms of postoperative morbidity and hospital stay.
If the tumor is not surgical assessable without significant morbidity and diagnostic scan are equivocal for necrosis, a biopsy may be warranted to rule out active disease. Since there is an increased risk of surgical wound dehiscence associated with bevacizumab, at our institution we delay surgery 4-6 weeks after the last administration of the drug.
Hyperbaric oxygen therapy in the management of brain necrosis
It has been estimated that one-third of cancer patients in the United states receive hyberbaric oxygen (HBO) for radiation induced injury (32). Normal tissue injury by radiation includes vascular damage, stromal fibrosis and the depletion of parenchymal as well as stems cells. Hyperbaric oxygen therapy has been shown to enhance angiogenesis in hypoxic or necrotic tissue, reduce fibrosis and mobilize stem cells within the irradiated tissue (33-37). HBO enhances the amount of dissolved oxygen in the plasma and thereby delivering more oxygen to the surrounding tissue. At a pressure of 3 atmospheres (304 kPa), dissolved oxygen approaches 60 mL/L of plasma whereas the sea-level concentration is only 3 mL/L. This difference in oxygen increase is sufficient to supply the resting tissue’s need without the contribution from oxygen bound hemoglobin. Therapeutic treatment pressures used inside a chamber are increased to 250-280 kPa. These pressures are equivalent to that experience in an underwater depth of 15-18 meters or 45-48 feet of water. Compared to monoplace chambers, the multiplace chambers are less claustrophobic and have less fire-risk since the high oxygen pressure is only released into the tight fitting patient mask and not the room itself. The multi-place chambers also have less risk of cross infection and assistants can enter the chamber t if a patient problem should arise. The duration of a daily treatment is typically less than 120 minutes but can vary from 45-300 min. A typical treatment course is 20 sessions. However, courses may be repeated depending on the patient’s response. For RN injuries, three consecutive courses for a total of 60 sessions have been given in our patient population.
Side effects of hyperbaric oxygen are often mild and reversible. Severe life threatening side effects are rare. Severe central nervous system symptoms including seizures occur in 1-2%, symptomatic reversible barotrauma in 15-20%, pulmonary symptoms in 15-20% and reversible myopia in 20% of patients treated (38). Myopia is the most common side effect due to the oxygen toxicity to the lens and, although reversible, can last for weeks to months.
In the largest series to date using HBO for RN, Gesell and her colleagues reported on the outcome in 29 patients (39). Objective neurologic exam improvement was observed in 58% of these patients while the need for steroids was reduced 69%. Other authors have seen similar results with a reduction of the size of necrosis on subsequent imaging studies for both SRS treated malignant tumors and AVMs (40-42). All in all, 65 patients in combined reports resulted in a 68% neurologic improvement following HBO for brain parenchyma radiation induced injury.
Although there is an expressed concern regarding reactivation of dormant malignant cells following HBO, there has been no observable recurrence documented in any of the literature (32,43). Although concern is warranted, the numbers are expected to be small.
Systematic reviews on HBO and cancer have concluded that the use of HBO in patients with malignancies is an option for corticosteroid refractory RN particularly if surgery is not an option to remove the lesion.
A proposed guideline for the treatment of brain necrosis from SRS
In summary, corticosteroids, surgery, bevacizumab and HBO are all possible good treatment options for symptomatic RN. The option selected should provide the patient with resolution of neurologic systems with the least toxicity and invasiveness. A suggested treatment guideline we use at our institution is as follows:
- Patients who have received SRS for brain metastases are followed with serial MRI scans every 2-3 months to assess disease response to radiation treatment and to rule out the interim development of new metastases. All brain MRI scans are obtained with and without contrast and include perfusion/diffusion.
- If the MRI scan reveals a probable necrotic lesion and the patient is asymptomatic, observation alone is advised with a repeat scan at 2-3 months intervals.
- If the MRI scan reveals probable RN and the patient is symptomatic, the lowest dose of corticosteroids (i.e., dexamethasone) can be offered for control of neurological symptoms. If the corticosteroids are tolerated, they can be continued until the lesion heals. If corticosteroids are not tolerated and the neurological symptoms from edema continue other treatment interventions should be considered.
- Patients who become refractory or intolerant to corticosteroids are discussed at tumor board. The prior radiation course, timing of new neurologic symptoms and changes on the MRI scan are review. Spectroscopy or a brain PET CT maybe obtained. If both sets of imaging are equivocal and the lesion is assessable, surgical resection can be offered to remove the mass. If removing the mass subjects the patient to unreasonable surgical risks, a biopsy might be attempted. The purpose of the biopsy is to distinguish between RN vs. active tumor so the appropriate treatment can be administered.
- If the patient is not eligible for surgery or the lesion does not cause significant mass effect, bevacizumab or hyperbaric oxygen therapy can be offered. If one therapy is not effective the other can be tried.
Fortunately, symptomatic RN is rare and many treatment options exist. Indeed, all the methods discussed in this article we have found effective in the treatment of SRS induced brain necrosis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “SBRT/SRS in Radiation Research”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.07.05). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. SV served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Flickinger JC, Lunsford LD, Kondziolka D, et al. Radiosurgery and brain tolerance: an analysis of neurodiagnostic imaging changes after gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys 1992;23:19-26. [PubMed]
- Voges J, Treuer H, Sturm V, et al. Risk analysis of linear accelerator radiosurgery. Int J Radiat Oncol Biol Phys 1996;36:1055-63. [PubMed]
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000;47:291-8. [PubMed]
- Petrovich Z, Yu C, Giannotta SL, et al. Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg 2002;97:499-506. [PubMed]
- Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys 2006;64:419-24. [PubMed]
- Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996-1001. [PubMed]
- Nedzi LA, Kooy H, Alexander E 3rd, et al. Variables associated with the development of complications from radiosurgery of intracranial tumors. Int J Radiat Oncol Biol Phys 1991;21:591-9. [PubMed]
- Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional analysis of complication outcomes after arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys 1999;44:67-74. [PubMed]
- Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011;6:48. [PubMed]
- de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 2004;63:535-7. [PubMed]
- Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res 2006;12:4738-46. [PubMed]
- Chamberlain MC. Pseudoprogression in glioblastoma. J Clin Oncol 2008;26:4359-author reply 4359-60. [PubMed]
- Jacobs AH, Kracht LW, Gossmann A, et al. Imaging in neurooncology. NeuroRx 2005;2:333-47. [PubMed]
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys 1980;6:1215-28. [PubMed]
- Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv 2004;4:273-84. [PubMed]
- Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000;217:377-84. [PubMed]
- Hygino da Cruz L Celso Jr, Rodriguez I, Domingues RC, Galheigo, D. Fatterpekar, G Pseudoprogression and Pseudoresponse: What we should know about the newly therapeutic approach of brain tumor and its effects . ASNR 2012 Poster.
- Asao C, Korogi Y, Kitajima M, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 2005;26:1455-60. [PubMed]
- Sundgren PC, Fan X, Weybright P, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 2006;24:1131-42. [PubMed]
- Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 2009;30:552-8. [PubMed]
- Bisdas S, Naegele T, Ritz R, et al. Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol 2011;18:575-83. [PubMed]
- Barajas RF, Chang JS, Sneed PK, et al. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2009;30:367-72. [PubMed]
- Sundgren PC. MR spectroscopy in radiation injury. AJNR Am J Neuroradiol 2009;30:1469-76. [PubMed]
- Ricci PE, Karis JP, Heiserman JE, et al. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 1998;19:407-13. [PubMed]
- Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res 2004;10:3342-53. [PubMed]
- Nonoguchi N, Miyatake S, Fukumoto M, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol 2011;105:423-31. [PubMed]
- Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 2007;67:323-6. [PubMed]
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011;79:1487-95. [PubMed]
- Sadraei NH, Dahiya S, Chao ST, et al. Treatment of Cerebral Radiation Necrosis With Bevacizumab: The Cleveland Clinic Experience. Am J Clin Oncol 2013; [Epub ahead of print]. [PubMed]
- Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol 2013;15:1257-63. [PubMed]
- Narita Y. Drug review: Safety and efficacy of bevacizumab for glioblastoma and other brain tumors. Jpn J Clin Oncol 2013;43:587-95. [PubMed]
- Feldmeier JJ. Hyperbaric oxygen therapy and delayed radiation injuries (soft tissue and bony necrosis): 2012 update. Undersea Hyperb Med 2012;39:1121-39. [PubMed]
- Marx RE. Radiation injury to tissue. In: Kindwall EP, ed. Hyperbaric Medicine Practice, Second Edition. Flagstaff, Best Publishing, 1999:665-723.
- Feldmeier JJ, Davolt DA, Court WS, et al. Histologic morphometry confirms a prophylactic effect for hyperbaric oxygen in the prevention of delayed radiation enteropathy. Undersea Hyperb Med 1998;25:93-7. [PubMed]
- Feldmeier JJ, Jelen I, Davolt DA, et al. Hyperbaric oxygen as a prophylaxis for radiation-induced delayed enteropathy. Radiother Oncol 1995;35:138-44. [PubMed]
- Goldstein LJ, Gallagher KA, Bauer SM, et al. Endothelial progenitor cell release inot circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells 2006;24:2309-18. [PubMed]
- Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest 2007;117:1249-59. [PubMed]
- Leach RM, Rees PJ, Wilmshurst P. Hyperbaric oxygen therapy. BMJ 1998;317:1140-3. [PubMed]
- Gesell LB, Warnick R, Breneman J, et al. Effectiveness of hyperbaric oxygen for the treatment of soft tissue radionecrosis of the brain. Presented at the 35th Annual Undersea and Hyperbaric Medical Society Scientific Meeting. USA, 2002.
- Hart GB, Mainous EG. The treatment of radiation necrosis with hyperbaric oxygen (OHP). Cancer 1976;37:2580-5. [PubMed]
- Leber KA, Eder HG, Kovac H, et al. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg 1998;70:229-36. [PubMed]
- Wang PC, Tu TY, Liu KD. Cystic brain necrosis and temporal bone osteoradionecrosis after radiotherapy and surgery in a patient of ear carcinoma. J Chin Med Assoc 2004;67:487-91. [PubMed]
- Feldmeier JJ. Hyperbaric oxygen: does it have a cancer causing or growth enhancing effect. In: Proceedings of the Consensus Conference sponsored by the European Society for Therapeutic Radiology and Oncology and the European Committee for Hyperbaric Medicine. Portugal 2001:129-146.


