CyberKnife stereotactic body radiotherapy and CyberKnife accelerated partial breast irradiation for the treatment of early breast cancer
Introduction
Phase I and II studies and some preliminary Phase III studies have challenged the standard of care [fully fractionated post-lumpectomy whole breast radiation therapy (WBRT)] for patients with early-stage breast cancer by delivering radiation to a restricted breast volume in fewer (i.e., 10 vs. 25) high-dose fractions, a technique known as accelerated partial breast irradiation (APBI) (1-3). Unlike WBRT, APBI limits the radiation to the region around the tumor bed in the hopes of reducing toxicity while maintaining equivalent cancer control rates. A more extreme form of APBI, stereotactic body radiotherapy (SBRT), aims to complete treatment in as few as five sessions. Here we describe, in a single report, our independent experiences using the CyberKnife System (Accuray Incorporated, Sunnyvale, CA, USA) for the delivery of APBI and SBRT to patients undergoing breast-conserving therapy.
The pathological argument for APBI
Poorer resolution mammography, non-universal pathologic margin standards, elementary radiation equipment and a naive bias toward a belief that cancer spreads broadly through the breast understandably resulted in post-lumpectomy WBRT becoming the early standard of care in breast conservation therapy (4). However, published data documents that 90% of breast cancer recurrences in women with early stage disease (stage 0-III) treated with lumpectomy with clear 2 mm or greater margins occur within 10 mm of the resection cavity (5-9). Others have shown 65-100% of breast cancer recurrences after conservative surgery and WBRT are in the same quadrant as the initial tumor and have the same histology as the primary tumor (10-12). Even without adjuvant radiotherapy, recurrence is located within the region of the tumor bed in the vast majority of cases (4,13-15). Because whole breast irradiation is not without side effects (16), radiation oncologists now question if it is necessary to treat the entire breast following a lumpectomy in all cases. Since side effects are related to fraction size and volume of normal tissue irradiated, reducing the volume is postulated to lower the risks. Also, by reducing the volume of normal tissue included within the radiation treatment field, the dose per fraction can be higher and overall treatment times reduced. Indeed, current APBI is commonly delivered in 5-10 fractions over 1-2 weeks.
APBI techniques
Interstitial multi-catheter brachytherapy
The oldest APBI technique, with the most published experience, is interstitial multi-catheter brachytherapy. Excellent control rates and acceptable toxicities are well documented with multi-catheter brachytherapy (3,8). Unfortunately, the procedure is invasive, carries the risk of infection and, similar to other multi-catheter brachytherapy techniques, is complex to perform. MammoSite (Proxima Therapeutics, Inc., Alpharetta, GA, USA) brachytherapy is a more user-friendly technique in which a single balloon is placed in the lumpectomy cavity. Although many have described the procedure as more comfortable for the patient compared to the multi-catheter approach, the balloon may not fit an irregularly shaped cavity or cannot be used if its placement is too close to the skin or chest wall. In addition, the catheter entry point is a source for infection requiring prophylactic antibiotics. On the other hand, a report from the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial reports a 91% good-to-excellent cosmetic result at a mean follow-up of 54 months in the treatment of 1,449 women with early breast cancer (17).
Intra-operative radiotherapy (IORT)
IORT is an elegant and efficient treatment approach to APBI, delivered at the time of the lumpectomy. The main criticism of this technique is that the final pathologic review of the specimen occurs a day or more after the treatment has been delivered prohibiting the re-excision in patients with a positive surgical margin. Nevertheless, IORT has been delivered to more than 5,000 patients in the TARGIT-A trial and in the Eliot Trial. Veronesi et al. (18) reported the outcomes of 1,822 patients who underwent breast conservation surgery and IORT. At 36 months mean follow-up, the local recurrence was 2.3%, local liponecrosis toxicity 4.2% and fibrosis 1.8%.
External beam techniques
Three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT) have gained popularity for early breast cancer patients seeking APBI. Both techniques are available at most radiation facilities and, unlike the brachytherapy modalities, are non-invasive. The disadvantage, however, is that the delivery of the beam is not as accurate. To compensate for the set-up uncertainty and respiratory motion during treatment, a larger margin to cover the 10-mm minimum risk area surrounding the cavity is required. Unfortunately this margin can result in greater coverage of normal structures such as the lung, chest wall and skin, and the heart particularly for left-sided lesions. Indeed, recent publications have shown greater toxicities with unacceptable cosmesis in women who elected a 3D-CRT or IMRT, APBI approach (19,20). In 5-year follow-up from a single-institution trial, Liss et al. reported a long-term rate of fair-to-poor cosmesis of 26.7% (21).
Disease control for APBI is promising. A recent study reported 5-year follow-up of patients stratified by risk according to the criteria established by the National Surgical Adjuvant Breast And Bowel Project (NSABP) B39/Radiation Therapy Oncology Group (RTOG) 0413 trial (in which women with early breast cancer are randomized to WBRT vs. APBI). In this study patients were treated with either MammoSite or multi-catheter HDR brachytherapy. No significant differences in tumor control rate (97.8% vs. 93.6%) or overall survival (92.1% vs. 89.5%) between low and high risk groups were obtained (22).
Stereotactic body radiotherapy and APBI
SBRT brings together the potential benefits of breast brachytherapy APBI with the non-invasiveness of external beam radiation therapy. SBRT delivers a highly conformal dose that mimics the dosimetry of a breast brachytherapy implant. The CyberKnife is a frameless robotic stereotactic radiosurgery system which provides image-guidance for continuous tracking of respiratory target motion and automatic correction of beam aim in real-time as the patient breathes. This results in dose placement accuracy to within about a millimeter for moving targets (23), which allows uncertainty margins to be very narrow, thus making it easier to keep doses to organs at risk low. In a treatment planning study researchers at the University of Texas Southwestern Medical compared CyberKnife SBRT, APBI and 3D-CRT treatment plans. They noted that the SBRT, APBI treatment plans achieved highly conformal target coverage and reduced the dose to nearby organs at risk relative to 3D-CRT plans (24). At Fox Chase Cancer Institute, a similar treatment planning comparison concluded that the CyberKnife’s more conformal dose could result in reduced toxicity by a reduction in dose to surrounding breast tissue (25) and patient movement including respiration (26).
CyberKnife APBI/SBRT: treatment methods
Twenty-one patients at Swedish Medical Center (Swedish) and 26 at Winthrop University Hospital (Winthrop) were treated. Two Swedish patients were treated in a 5-fraction regimen, but due to insurance limitations most patients were treated using a 10-fraction APBI protocol. Winthrop patients were treated with 5-fraction SBRT as part of an IRB-approved protocol. Patient selection criteria closely followed the American Society for Radiation Oncology (ASTRO) consensus statement for “suitable” or “cautionary” candidates (27). Women older than 45 years of age with Tis, T0, T1, T2 non-lobular carcinomas less than 3 cm, with negative margins (>2 mm) and lymph nodes, were eligible (Table 1). APBI was initiated within 9 weeks of the patient’s last breast cancer surgery.
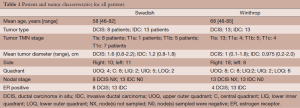
Full table
Fiducial implantation
At Swedish 4-5 gold fiducials were placed in the walls of the cavity at the time of the lumpectomy to allow CyberKnife tracking of respiratory motion. For 25 Winthrop patients fiducial markers were placed by the treating radiation oncologist under image guidance on a CT simulator with coordinate placement determined by the physics/dosimetry staff for optimal location. One patient had fiducial markers placed by the surgeon.
Treatment planning, immobilization
At Swedish non-contrast computed tomographic (CT) scans (1.0-mm slice thickness) were acquired with the patient wearing a support bra and placed in an alpha cradle with arms at her side supported below the chest. The CT images started at the mandible and extended several centimeters below the inframammary fold. Non-contrast magnetic resonance images (MRI) were fused to CT when the lumpectomy cavity was ill-defined on CT due to the adjacent breast tissue density or artifact scatter from the fiducials. The lumpectomy cavity was best delineated on the T2 axial or STIR MRI images. The fiducials were seen on the 2dT2 (STAR) sequence and used to verify the correctness of the fusion with the CT. At Winthrop similar practices were followed except patients were immobilized either using a thermoplastic cast across the chest with a hole removed around the areola to facilitate repositioning, or in an alpha cradle with the breast in its natural position. At Winthrop treatment planning was based on CT imaging only.
Treatment volumes, dose and fractionation
The clinical target volume (CTV) was defined as the lumpectomy cavity plus 15 mm. The planning target volume (PTV) was defined as the CTV plus a 2-mm margin while ensuring a 5-mm sparing distance from the skin and chest wall. Also, a field within a field was created to force the dose maximum into the lumpectomy cavity. The 2-mm CTV margin was added to accommodate for the possible tracking error of the fiducials. No additional volumes were considered necessary to account for variability in day-to-day set-up or patient mobility.
At Swedish, the first two patients were treated with an SBRT regimen of 5 fractions of 5 Gy each. Difficulty securing insurance for SBRT forced adoption of a 10-fraction APBI approach. Patients initially received 34 Gy in 10 fractions delivered to the PTV, prescribed to the 65-75% isodose. After 12 patients were treated without toxicity, the peripheral dose was increased to 36 Gy in 10 fractions. One patient’s overall treatment time was decreased to 6 fractions because of co-morbidities and a difficult commute to the center. The dose at the cavity wall was 38.5 Gy or greater. Treatment was typically performed twice daily, although when scheduling conflicts arose we extended the treatment time but ensured its completion within 2 weeks. At Winthrop all patients were treated under an SBRT protocol delivering 30 Gy in 5 equal, 6-Gy fractions to a median prescription isodose of 71%. These isodoses were chosen to allow for a more rapid fall-off of dose beyond the target volume, thus more closely emulating HDR brachytherapy treatment. Treatment times averaged 46 min, ranging from about 36 to 55 min.
The dose constraints at both sites were based upon the NSABP/RTOG protocol (Table 2). For very medial inner quadrant or lower inner quadrant lesions, acceptance of a higher dose point, not volume, was allowed for the contra-lateral breast, the heart and lung. The volumes allowable for these structures were well below the acceptable limits by one third to one half. As an example, the largest contra-lateral breast point in our series was 8 Gy. The volume of the breast that received 0.5 Gy, however, was only 1.5%.
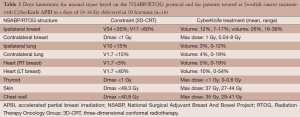
Full table
In addition to examination of dosimetry, acute and late toxicity, and disease control, cosmesis was judged using the Harvard cosmesis scale at multiple time points post-treatment. An excellent outcome was defined as “minimal or no difference” in appearance and good cosmesis was defined as “a slight difference”. Fair or poor cosmesis defined as “obvious differences...involving a quarter or less of the breast” or “as marked change involving more than a quarter of the breast tissue”.
Results
Swedish
The mean PTV for the whole group was 114 cm3 (range, 39-241 cm3) and mean percent isodose prescription line was 70% (range, 65-76%). The mean percent of the whole breast reference volume receiving 100% and 50% of the dose (V100 and V50) was 12% (range, 7-17%) and 26% (range, 16-39%), respectively. Treatment plans generally met dose constraints, although in a few cases upper ranges exceeded some constraints [see Table 2; for a fuller account of APBI dosimetry see (28)]. Dosimetry for the patients at Winthrop (not shown) did not differ substantially from that depicted in Table 2. The beam number mean was 151 (range, 95-250). Two patients not counted among the 21 treated were simulated but not treated. One had an enlarging seroma that twice altered the positions of the fiducials from the planning CT. The second patient had poor breast integrity which also resulted in changes in fiducial position. Both patients were sent for whole breast irradiation.
At a mean follow-up of 31 months (range, 6-57 months), no breast cancer recurrence has been identified. Acutely, minimal erythema involving a small portion of the breast was reported by two patients and minimal fatigue was observed by half of the patients treated. No treatment was given for these acute toxicities which subsided by 2 and 3 weeks respectively. One patient had minor pain at the lumpectomy site at 10 months since treatment. One patient has palpable non-painful firmness at the lumpectomy site but the shape of the breast was excellent and skin fibrosis minimal. The size, shape and texture of a patient’s treated breast was compared to the breast’s original appearance after surgery and from pictures taken at the time of simulation. Cosmetic outcome were excellent or good in all 21 patients treated.
Winthrop
The mean PTV for the whole group was 113 cm3 (range, 25-274 cm3). The mean percent of the of the whole breast reference volume receiving 100% and 50% of the dose (V100 and V50) was 14% and 29%, respectively. The median number of beams was 122 (range, 89-187).
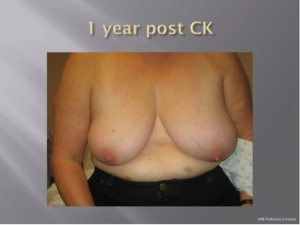
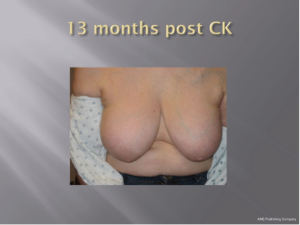
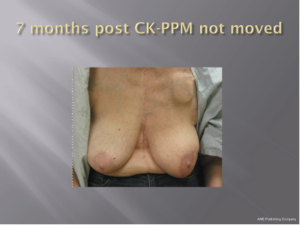
With a median follow-up of 21 months (range, 7-39 months) all 26 patients (100%) remain locally controlled with no evidence of disease following treatment. Acutely, RTOG Grade 1 dry skin desquamation occurred in 1 of 25 patients. The cosmesis was good-excellent in all 25 patients using the Harvard cosmesis scale. Figures 1,2,3 show examples of maintained breast cosmesis.
Discussion
Based on these preliminary results we are optimistic that with stereotactic tracking ability and a low prescription isodose, issues involving patient motion, set-up reproducibility and toxicity are of less concern with CyberKnife APBI than for patients receiving 3D-CRT. Indeed, the PTV is similar to that seen in patients treated with multi-catheter or balloon catheter brachytherapy. The mean ipsilateral breast volumes receiving 100% and 50% of the prescribed dose were less than half that allowable in the NSABP/RTOG study. Without any observable acute side effects and excellent/good cosmetic outcomes, and the fact that normal tissue constraints are easily met, we conclude that the CyberKnife provides a suitable non-invasive approach for delivering APBI for women with early breast cancer.
Disadvantages of this approach include the need for fiducial-based tracking. The cooperation of lumpectomy surgeons or straightforward fiducial implantation procedures can lessen the difficulty this poses for physicians and patients. The fiducials array must also stray minimally from their positions during planning CT scanning to allow accurate tracking in all six dimensions, which puts a premium on effective implantation and patient setup procedures. It also requires that changes in breast morphology during treatment be minimal, which can usually be achieved given the short treatment times, but note again the unusual circumstances with the patient from Swedish. In addition, treatment session times are considerably longer than those required for conventionally fractionated WBRT. This is usually not a difficult tradeoff for patients, however, as 5-10 sessions are generally much more convenient than 25. Although at Swedish we were compelled to use a 10-fraction APBI approach, we believe that 5-fraction SBRT with the CyberKnife is feasible and is likely to be a highly convenient, effective adjuvant to lumpectomy with low toxicity and very good to excellent cosmetic results. Still, long-term follow-up from well-controlled prospective studies is required to make strong claims about the value of the approach. In addition, as is clear from this report, sites evaluating APBI/SBRT with the CyberKnife are developing different treatment planning methods, doses and fractionation, and workflows; some attention to optimizing practices would be necessary to develop multi-institutional trials.
Conclusions
CyberKnife SBRT/APBI is currently under investigation at many centers for the treatment of early breast cancer. SBRT/APBI offers patients radiation treatment in a much shorter time than WBRT and without the invasiveness of a brachytherapy implant. In-breast tumor recurrence is the primary endpoint of SBRT/APBI studies. Quality of life (QOL) endpoints are also measured and include cosmesis, fatigue, breast-related symptoms and perceived convenience of care. Continued follow-up is needed to confirm that SBRT/APBI goals measured in these ways are met. As a result, all centers considering CyberKnife SBRT/APBI for their patients are encouraged to submit to national or Investigational Review Board-approved studies. Off-study patients should be treated according to the ASTRO eligibility guidelines published in 2009 for women considering ABPI for early breast cancer.
Acknowledgments
The authors thank David Schaal, PhD, of Accuray Incorporated, for helpful comments and Astrid Sanchez for accurate and timely data management on the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “SBRT/SRS in Radiation Research”. The article has undergone external peer review.
Conflicts of Interest: Dr. Vermeulen has no conflicts of interest; Dr. Haas has received speaker’s honoraria from Accuray Incorporated.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB). Written informed consent was obtained from every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Formenti SC, Truong MT, Goldberg JD, et al. Prone accelerated partial breast irradiation after breast-conserving surgery: Preliminary clinical results and dose–volume hist analysis ogram. Int J Radiat Oncol Biol Phys 2004;60:493-504. [PubMed]
- Keisch M, Vicini F, Kuske RR, et al. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 2003;55:289-93. [PubMed]
- Vicini FA, Baglan KL, Kestin LL, et al. Accelerated treatment of breast cancer. J Clin Oncol 2001;19:1993-2001. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Clark RM, McCulloch PB, Levine MN, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst 1992;84:683-9. [PubMed]
- Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol 1990;8:113-8. [PubMed]
- Rosen PP, Fracchia AA, Urban JA, et al. “Residual” mammary carcinoma following simulated partial mastectomy. Cancer 1975;35:739-47. [PubMed]
- Vicini FA, Kestin L, Chen P, et al. Limited-field radiation therapy in the management of early-stage breast cancer. J Natl Cancer Inst 2003;95:1205-10. [PubMed]
- Vicini FA, Kestin LL, Goldstein NS. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: a pathologic analysis. Int J Radiat Oncol Biol Phys 2004;60:722-30. [PubMed]
- Fisher ER, Sass R, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). II. Relation of local breast recurrence to multicentricity. Cancer 1986;57:1717-24. [PubMed]
- Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys 1990;19:833-42. [PubMed]
- Liljegren G, Holmberg L, Adami HO, et al. Sector resection with or without postoperative radiotherapy for stage I breast cancer: five-year results of a randomized trial. Uppsala-Orebro Breast Cancer Study Group. J Natl Cancer Inst 1994;86:717-22. [PubMed]
- Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 2002;20:4141-9. [PubMed]
- Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus Tamoxifen with or without Irradiation in Women 70 Years of Age or Older with Early Breast Cancer. N Engl J Med 2004;351:971-977. [PubMed]
- Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med 1993;328:1587-91. [PubMed]
- . Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. N Engl J Med 1995;333:1444-55. [PubMed]
- Vicini F, Beitsch P, Quiet C, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2011;79:808-17. [PubMed]
- Veronesi U, Orecchia R, Luini A, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat 2010;124:141-51. [PubMed]
- Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys 2010;76:71-8. [PubMed]
- Hepel JT, Tokita M, MacAusland SG, et al. Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys 2009;75:1290-6. [PubMed]
- Liss AL, Ben-David MA, Jagsi R, et al. Decline of cosmetic outcomes following accelerated partial breast irradiation using intensity modulated radiation therapy: results of a single-institution prospective clinical trial. Int J Radiat Oncol Biol Phys 2014;89:96-102. [PubMed]
- Patel RR, Christensen ME, Hodge CW, et al. Clinical outcome analysis in “high-risk” versus “low-risk” patients eligible for national surgical adjuvant breast and bowel B-39/radiation therapy oncology group 0413 trial: five-year results. Int J Radiat Oncol Biol Phys 2008;70:970-3. [PubMed]
- Hoogeman M, Prévost JB, Nuyttens J, et al. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: assessment by analysis of log files. Int J Radiat Oncol Biol Phys 2009;74:297-303. [PubMed]
- Heinzerling JH, Ding C, Ramirez E, et al. Comparative cose-volume analysis for Cyberknife and 3D conformal partial breast irradiation treatment of early stage breast cancer. Int J Radiat Oncol Biol Phys 2010;78:S825-6.
- Fan J, Hayes S, Freedman G, et al. Planning the breast boost: Dosimetric comparison of Cyberknife, photo mini tangents, IMRT and electron techniques. Int J Radiat Oncol Biol Phys 2010;78:S788-S789.
- Kilby W, Dooley JR, Kuduvalli G, et al. The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat 2010;9:433-52. [PubMed]
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [PubMed]
- Vermeulen S, Cotrutz C, Morris A, et al. Accelerated Partial Breast Irradiation: Using the CyberKnife as the Radiation Delivery Platform in the Treatment of Early Breast Cancer. Front Oncol 2011;1:43. [PubMed]