
Should trastuzumab be administered concomitantly with anthracycline in women with early, HER2-positive breast cancer?
The monoclonal antibody trastuzumab, the first anti-human epidermal growth factor receptor 2 (HER2) therapy introduced in the clinic, has represented a major step forward in the treatment of breast cancer (1). HER2 gene amplification, which almost invariably results in overexpression of its product, a transmembrane tyrosine-kinase receptor, is found in about 15% of breast cancers. This abnormality drives an aggressive clinical phenotype characterized, in the absence of specific targeting, by increased risk of relapse after surgery of localized disease, tendency to spread to distant organs with frequent visceral and central nervous system involvement, resistance to endocrine manipulation and short survival times in patients with metastatic disease (2). HER2-targeting with trastuzumab has resulted in a dramatic improvement in the life expectancy of women with metastatic disease and, with its introduction in adjuvant programs for operable disease, in a significant increase in cure rate. While newer anti HER2-agents are improving the clinical outlook of HER2-positive breast cancer patients beyond what was once hardly conceivable, much of the critical information on how to integrate anti HER2-therapy in the management of these patient comes from the early experiences with trastuzumab. As single agent, trastuzumab showed modest activity in women with HER2-positive metastatic breast cancer (1). Response rates ranging from 15% to 35% were observed according to the load of previous treatments for metastatic disease. However, as preclinical studies suggested, and for reasons that are still not completely understood, the full potential of HER2-targeting with this monoclonal antibody could be exploited by combining it with conventional chemotherapy (3). This rationale was explored in a pivotal randomized trial that led to the approval of the monoclonal antibody trastuzumab (4). In this study, women with HER2 positive breast cancer were randomized to chemotherapy with or without concomitant trastuzumab as first line therapy for metastatic disease. The chemotherapy schema was different according to whether patients had received anthracyclines in the adjuvant setting. In case of no prior exposure, the chemotherapy schema consisted of AC (doxorubicin 60 mg/m2 or epi-doxorubicin 75 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for six or more cycles). Those who had received an anthracycline in the adjuvant setting received paclitaxel at the dose of 175 mg/m2 every 3 weeks for 6 or more cycles. This study provided evidence that trastuzumab could improve response rate, progression-free survival (PFS) and overall survival (OS), compared with chemotherapy alone. However, and unexpectedly, trastuzumab resulted associated with significant cardiac dysfunction, with a 27% incidence of left ventricular ejection fraction (LVEF) depression, including a 16% incidence of heart failure (New York Heart Association Class III and IV) in the anthracycline-containing arm. Notably, the corresponding figures in the AC alone arm were 8% and 3%, respectively. Although significantly less frequently, cardiac toxicity was observed also in the paclitaxel plus trastuzumab arm (overall 13%, NYHA class III and IV 2%). These findings prompted a systematic retrospective analysis of all the trastuzumab trials conducted at the time and research to elucidate the role of HER2 in cardiac function (5). Trastuzumab was confirmed to induce LVEF depression. However, differently from anthracycline-induced cardiomyopathy which is characterized by irreversible myofibrillar damage, trastuzumab effect was mostly reversible upon withdrawal of this antibody (5). Molecular biology studies revealed that HER2 is involved in embryonic cardiac development (6). Furthermore, the epidermal growth factor receptor (EGFR) family, which includes HER2, is involved in repairing the oxidative damage related to anthracycline exposure (7). Therefore, pharmacological inhibition of the physiological function of HER2 in the heart could account for the toxicity observed when anthracycline and trastuzumab were administered together (7). The lessons from these initial experiences have been carried forward over the years up to present time, having had a profound influence on the design of any anti HER2 therapeutic strategy. Screening for pre-existing cardiac conditions that could predispose patients to cardiac toxicity, avoidance of concomitance with anthracyclines, regular cardiac monitoring and proactive cardiac pharmacologic intervention to support LVEF are considered both in clinical trial design and in the current clinical practice, regardless of the anti HER2-compound used (8). Tackling trastuzumab-related cardiac toxicity was a relevant issue when trastuzumab was studied in the adjuvant setting, where anthracycline are an important component of the chemotherapy regimens. In fact, a meta-analysis of studies comparing anthracycline-based vs. cyclophosphamide, methotrexate, 5-fluorouracil (CMF)-like adjuvant treatments found that the major efficacy of the former regimens was restricted to women with HER2-positive breast cancer (9). Beyond strict cardiac inclusion criteria, trialists dealing with trastuzumab-based experimental arms used different approaches (8): administering trastuzumab after anthracyclines and concomitantly with taxanes, developing anthracycline-free adjuvant chemotherapy regimens, where trastuzumab could be administered concomitantly with the complete chemotherapy program, or administering trastuzumab after the completion of chemotherapy, regardless of the regimen used. Because partial or no overlap between trastuzumab and chemotherapy could be seen as a potential limitation to the full exploitation of trastuzumab-based therapy, a number of authors tried to evaluate the feasibility of anthracyclines, either conventional or liposomal, administered concomitantly with trastuzumab in the metastatic setting (10). While these experiences could not convincingly demonstrate the safety and convenience of these regimens, the results of a small study conducted ad M.D. Anderson Cancer Center caused quite a stir in the field (11). Women with HER2-positive operable breast cancer were randomized to neoadjuvant chemotherapy consisting of four cycles of paclitaxel (225 mg/m2 as a 24-hour continuous infusion every 3 weeks) followed by four cycles of FEC75 (5-fluorouracil 500 mg/m2, epi-doxorubicin 75 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks for four cycles) with or without concomitant trastuzumab (24 weeks of treatment). The study was prematurely closed after the accrual of just 42 patients because of an unprecedented rate of pCR in the “all concomitant” trastuzumab and chemotherapy arm of 66.7%, compared with 25% in the chemotherapy alone arm (Table 1). Subsequent follow-up of patients treated with that regimen showed a high rate of freedom from disease progression (20). Most importantly, authors did not find a strong signal towards cardiac toxicity. After the MD. Anderson study other groups studied concomitant regimens confirming high rates of pCR and an acceptable profile of cardiac toxicity (Table 1) (12-14,16,17). Furthermore, a small, but provocative study from Finland used an “all concomitant” adjuvant regimen of taxanes or vinorelbine followed by FEC and trastuzumab given for 9 weeks only (21). Hazard ratios for event-free survival (EFS) and OS were in the same range of those reported in the registration trials, where trastuzumab was not combined with anthracycline and given in general for one year. Also in this case, no signal for increased cardiac toxicity was observed in the trastuzumab arm of the study.
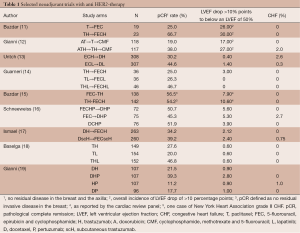
Full table
These results suggested revisiting the concept of full concomitance between trastuzumab and anthracycline-based therapy, as an approach to optimize the efficacy of trastuzumab in the setting of early breast cancer. One important consideration regarding the cardiac concerns associated with trastuzumab treatment is that patients in small trials of neoadjuvant chemotherapy may represent a selected population that only partially overlaps with the “real world” patients. Population based studies reveal that, because of frequent cardiac conditions which, by themselves, do not represent a contraindication to treatment, the incidence of cardiac toxicity despite all the precautions is higher than that reported in clinical trials (22). For all these reasons, whether increasing the potential efficacy of trastuzumab based regimens is worth the risk of giving it in concomitance with anthracyclines has remained an open issue until recently. A randomized study from the Institution that first showed the potentiality of the “all concomitant” approach has provided a convincing response (15). The Z1041 trial randomized a total of 282 women with early, HER2-positive breast cancer to either a sequential arm of four cycles of FEC75 followed by weekly paclitaxel (80 mg/m2/week for 12 weeks) plus 12 weekly administrations of trastuzumab (4 mg/kg loading dose, followed by weekly doses of 2 mg/kg) or to a concomitant arm of weekly paclitaxel (same schedule as above) followed by four cycles of FEC75 with weekly trastuzumab started with paclitaxel and administered for a total of 24 weeks. Upon completion of treatment patients were schedule to undergo surgery and, then, advised to continue trastuzumab for up to one year. The primary study end-point was pCR in the breast and the study was powered on the hypothesis that the concomitant schedule could increase the pCR rate by 20% or more, from an expected 25% in the sequential arm. Patients were meticulously selected on the basis of strict cardiac criteria, including no history of myocardial infarction, congestive heart failure (CHF), cardiomyopathy, or cardiac disease requiring drug treatment; severe conduction abnormality, valvular disease, cardiomegaly, ventricular hypertrophy on electrocardiography, or poorly controlled hypertension. The striking finding of this trial was that doubling the duration of trastuzumab and giving it in full concomitance with a taxane and anthracycline-based chemotherapy yielded a high pCR that was similar to that achieved by patients in the more conventional sequential arm, were trastuzumab duration was just a half (12 weeks) (Table 1). In fact, pCR rate in the latter arm was largely higher than expected. The study also confirmed the cardiac feasibility of trastuzumab with anthracyclines, with caveats to consider regarding the selection of patients (see above). The results of the Z1041 trial mirror those of the previously published TRYPHAENA trial (Table 1) (17), where HER2-inhibition consisted of trastuzumab and the other anti HER2 monoclonal antibody pertuzumab and confirm that whether anti HER2 therapy should be administered in full concomitance with a sequence of anthracycline and taxanes is no longer an issue. On a more general level, they also suggest that further manipulation of the classical ingredients of the HER2-therapy recipe (anthracycline, taxanes, concomitance and duration) will hardly result in an increase in pCR, and possibly, in cure rate. In fact, two studies using single agent chemotherapy and double HER2 targeting with either the tyrosine-kinase inhibitor lapatinib or pertuzumab showed impressive rates of pCR obtained after just 16 weeks of treatment (Table 1). In those two studies, an anthracycline-based regimen was administered after surgery, before resuming HER2-targeting. A relevant scientific issue would be assessing the role of further anthracycline therapy in those patients achieving pCR in the neoadjuvant setting. In fact, not all patients who achieve a pCR show long-term EFS, but for a proportion of them anthracycline and their fearsome general and cardiac toxicity could be possibly avoided. On the other hand, as a slightly better DFS and OS favouring AC followed by docetaxel plus trastuzumab over the anthracycline-free docetaxel, carboplatin and trastuzumab was observed in the Breast Cancer International Research Group (BCIRG) 006 trial (23). This suggests the existence of a subset of HER2 positive tumors that may be better cured with anthracycline-containing regimens combined with HER2 targeting.
For now, clinicians managing operable HER2-breast cancer patients at risk of relapse are reinforced in their prescribing patterns by the results of the Z1041 study. However, the real challenge, especially in the era of multiple HER2-targeting strategies, is to tailor treatment intensity to clinical and biological features of the tumor. There is increasing recognition that breast cancer in general, and HER2 positive breast cancer in particular can be further grouped on the basis of their molecular heterogeneity (24). Deciphering this heterogeneity has several obvious implications in the process of optimizing the toxicity/benefit and cost/effectiveness ratios of treatment for HER2-positive breast cancer. In this respect, considering that adjuvant anti HER2 therapy is increasingly offered to patients with small, node-negative, HER2 positive tumors, depotentiation of the chemotherapy component of programs should became a major focus for research. Once again, however, translational research is called into cause in the eternal quest for biomarkers that could help stratify patients according to the likelihood to derive benefit from a specific treatment approach. Unfortunately, the jury is still out for the most promising candidate biomarker of potential sensitivity to anthracycline, the topoisomerase 2 gene status or protein expression (25). Yet, further research in this direction should be pursued to define those patients for whom anthracycline could be safely omitted and those, conversely, for whom these drugs still represent a life-saving option.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Breast Cancer”. The article did not undergo external peer review.
Conflicts of Interest: I am member of the speaker’s bureau for Hoffmann La Roche S.p.A, and consultant and member of the speaker’s bureau for GlaxoSmithKline S.p.A. The author declares no conflict of interest.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Montemurro F, Valabrega G, Aglietta M. Trastuzumab-based combination therapy for breast cancer. Expert Opin Pharmacother 2004;5:81-96. [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [PubMed]
- Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999;18:2241-51. [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [PubMed]
- Telli ML, Hunt SA, Carlson RW, et al. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 2007;25:3525-33. [PubMed]
- Lee KF, Simon H, Chen H, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995;378:394-8. [PubMed]
- Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 2002;8:459-65. [PubMed]
- Rossi V, Redana S, Milani A, et al. Trastuzumab in the adjuvant setting: a practical review. Therapy 2011;8:161-77.
- Gennari A, Sormani MP, Pronzato P, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst 2008;100:14-20. [PubMed]
- Rayson D, Richel D, Chia S, et al. Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: current experience and future strategies. Ann Oncol 2008;19:1530-9. [PubMed]
- Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 2005;23:3676-85. [PubMed]
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377-84. [PubMed]
- Untch M, Loibl S, Bischoff J, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 2012;13:135-44. [PubMed]
- Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 2012;30:1989-95. [PubMed]
- Buzdar AU, Suman VJ, Meric-Bernstam F, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1317-25. [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-84. [PubMed]
- Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 2012;13:869-78. [PubMed]
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [PubMed]
- Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 2007;13:228-33. [PubMed]
- Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 2009;27:5685-92. [PubMed]
- Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012;104:1293-305. [PubMed]
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [PubMed]
- Montemurro F, Di Cosimo S, Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol 2013;24:2715-24. [PubMed]
- Press MF, Sauter G, Buyse M, et al. Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol 2011;29:859-67. [PubMed]


