A simulation based dosimetric study for a Kypho-IORT treatment using Intrabeam™
Introduction
The skeletal system is the third most frequent site of metastases (after lung and liver), with up to 30% of all cancers developing bone metastases (1). More specifically, patients with breast and prostate cancers suffer from bone metastases in up to 85% of the cases. Approximately 50% of all bone metastases are located in the spinal cord with pain, neurological dysfunction, and reduced activity resulting from hypercalcaemia and bone fractures identified as major complications (2).
A combination of surgical stabilization and radiotherapy is the current treatment standard for treating fractured vertebral bodies caused by spinal metastases. At the same time for spinal metastases indications a more local radiotherapy approach may be more appropriate (3) for certain patients from both a therapeutic and logistics’ point of view. Intraoperative radiation therapy (IORT) has therefore been suggested as an alternative strategy for radiation treatment in these patients either completely replacing the external beam therapy or the external boost only. Such local treatments require the use of lower photon energies compared to conventional external beam therapy (4-15 MV), and deliver a localized dose to the part of the spine closest to the metastases.
At present, no calculation tool allows to accurately determine the dose received during an irradiation using the Intrabeam™ system. In this context, the objective of this work is therefore to validate a patient specific Monte Carlo (MC) simulation based dose planning and evaluation system in a Kypho-IORT clinical perspective.
Such a system needs to take into consideration not only the source and patient specific characteristics but also the source location inside the patient during treatment. This should be taken into account in the dosimetric evaluation of an IORT procedure using the Intrabeam™. A framework of the integration of the source position recovered during surgery will be proposed within the same dose planning and evaluation platform.
Materials and methods
X-ray source description
Amongst other IORT devices the use of novel miniature X-ray generator like the Intrabeam™ system (Carl Zeiss Surgical, and Oberkochen, Germany) has been recently proposed. Intrabeam™ is based on a mobile miniaturized radiation source (XRS4™) which consists of a 10 cm long, 3.2 mm diameter tube that is attached to a larger housing, containing an electron gun and associated electronics. The tube is made primarily of molybdenum metal for magnetic shielding, except for the final 2 cm at the end of the tube that are made of beryllium, which acts as a transparent X-ray window. The entire probe is coated with a thin layer of chromium nitride (NCr) in order to render the device biocompatible. X-rays are created by forming and focusing an electron beam in the electron gun, accelerating the beam with a maximum power of 50 kV and 40 µA and allowing the beam to travel down the evacuated tube to strike a thin gold target of 0.5 µm that lines the inside end of the tube. Both Bremsstrahlung X-rays and characteristic line radiation are emitted from the tip of the tube and photon emission is isotropic. An internal radiation monitor (IRM) detects the part of the X-ray photons emitted in the direction of the cathode and records dose output in real-time. The IRM result is displayed on the treatment screen of the control terminal so that the operator knows what dose is being delivered at any time throughout treatment (4). Depending on the clinical application, various types and sizes of applicators can be attached to the X-ray source. For the kypho-IORT, a specific applicator design has been developed based on stainless steel tube with a plastic (ULTEM polyetherimide) tip to minimize the absorption of the radiation. The single use INTRABEAM Needle Applicator has a length of 94 mm and a diameter of 4.4 mm. Specially designed metallic sleeves (5 mm diameter, 6 cm length) are used to guide the electron drift with needle applicator around to be inserted in the patient.
GATE based model
A MC model of the Intrabeam™ has been previously developed with GATE (www.opengatecollaboration.org) v6.2 (Geant4 Application for Tomography Emission), based on Geant4 libraries (5). GATE was originally developed for positron emission tomography and single-photon emission computed tomography (CT) applications. A recent release (v6.0) allows performing simulations of external beam radiation therapy via the use of dose actors (6). A dose actor stores the absorbed dose and/or the deposited energy in a given volume into a 3D image according to the spatial position of the hits and takes into account the weight of the particles. A circular source of photons was simulated instead of accelerated electrons striking a gold target within the X-ray tube. Root software (version 5.34) was used to calculate the photon spectrum. GATE provides different models for particle tracking. Each model includes specific processes and energy cuts. The standard energy model, including electron ionization and multiple scattering above 1 keV, was selected for electron tracking. Below 1 keV, electrons were not tracked anymore and local deposited energy was thus considered. Indeed, the electron path does not exceed few microns in air at 1 keV, whereas in our simulations, the voxel dimensions were never less than 1×1×1 mm3. For photon tracking, the standard energy model of Geant4 was chosen: it takes into account the photon path from 1 keV to 1 TeV.
Kypho-IORT set-up
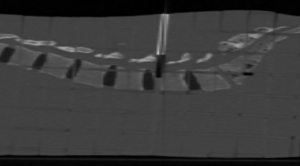
In order to simulate a real treatment of kypho IORT we used an anthropomorphic phantom called RANDO® which is constructed with a natural human skeleton cast material that is radiologically equivalent to soft tissue. To facilitate dose mapping, RANDO® phantoms are divided into 2.5 cm sections. Optional holes grids are drilled through the phantom’s soft tissue material allowing a variety of dosimeters to be used. In order to not make a hole in the phantom for the insertion of the applicator a RW3 plate of 0.5 mm width, cut in the shape of the body phantom was added. A hole was subsequently made in this phantom part in order to allow the insertion of the needle applicator to simulate a real kypho-IORT treatment (Figure 1).
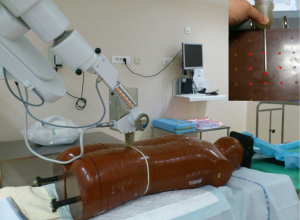
Dose measurements are then obtained by using thermoluminescents dosimeters (TLD) inserted in the holes on the slice close to the source, before and after the one where the needle applicator is inserted. Nine TLD were then placed inside the holes around the X-ray source in each RANDO slice. Square TLDs of LiF:Mg (3×3×1 mm3, TLD700) were used in this study because they are well suited for these measurements due to their near to tissue equivalence and small physical size.
After positioning the TLDs, the source was inserted in the applicator needle and in the phantom body in order to perform a real irradiation. A dose of 5.87 Gy at 8 mm was prescribed as specified by the manufacturer for this kind of treatment, which corresponds to a 94 sec long treatment.
CT acquisition
An X-ray CT acquisition of the phantom with the needle applicator inserted inside the body was performed in order to know exactly where the applicator was positioned inside the body. The CT image was also mandatory for computing dose with the MC simulation. Simulated dose results from GATE were then compared with measurements, using dose elements located at the same position as TLDs and with the same size as TLD sensitive volume (Figure 2).
Results
Dose comparison
Table 1 give results of the comparison between calculated dose rates (in Gy/min) from MC GATE simulations and measured dose rates using TLDs during the treatment. Table 1 gives the relative deviation between simulated and experimental results and GATE statistical uncertainties. Results are presented at the skin level and inside the phantom body. Distance between TLD measurements inside the body is 3 cm.
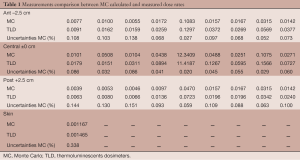
Full table
Dose distribution
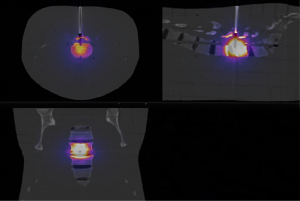
After simulation of the kypho-IORT treatment, CT images of the phantom including the applicator were integrated in the GATE macro in order to perform a MC simulation of the whole treatment. The dose distribution obtained in Figure 3 shows the dose deposited locally around the tip of the applicator in the vertebra.
Discussion and conclusions
In current clinical use, the INTRABEAM™ system delivers a single dose fraction of 5.87 Gy at 8 mm depth in the case of Kypho-IORT. At present, upon irradiation the single known dose is the dose prescribed, which corresponds to that delivered at 8 mm depth. The resulting total treatment time is based on the prescription parameters, the dose rate and measured depth dose curves in water. While this procedure is sufficiently precise for treating homogeneous water equivalent tissue it is not sufficiently accurate in cases of inhomogeneous tissue surrounding the applicator. No patient specific dosimetry is currently performed while the Euratom Directive 97/43 of 30 June 1997 states in Article 4 the requirement to perform dose optimization not only for each patient specific tumor but also for the surrounding tissues at risk. However, if the dose is not accurately known it cannot be subsequently optimized. Several factors come into play. Firstly, considering the 50 keV energy the dose gradient is so high that any measure is hampered by significant uncertainty related to the positioning accuracy of a detector. Secondly, considering that the low-energy spectrum of photons varies rapidly with depth, this rapid variation of the spectrum makes it difficult to measure (detector response is never independent of the beam spectrum). In addition, in order to accurately calculate the dose, this change of the spectrum with depth must be taken into account. On the other hand, for 50 keV, (considering an average energy of 20 keV to the surface of the applicator), water is not a tissue equivalent material. It therefore seems essential to perform dose calculations, not using a homogeneous water phantom but from CT images taking into account the spatial distribution and associated tissue densities for each patient. Results given in Table 1 show a good fit with experimental measurement. The mean absolute deviation between simulation and measurement dose rate was 0.014±0.009, 0.075±0.319 and 0.006±0.008 Gy/min for the anterior slice the central one and the posterior slice respectively. the differences between simulation and measurement are not significant at this low energy; Also the use of only one thermoluminescent dosimeter per measurement is not enough to avoid effect of variation mainly at this low energies, but the phantom’s holes do not have size for more than one TLD.
As a conclusion, a simulation based dosimetry framework is described and proposed for intraoperative treatment of vertebral metastases. The next step will involve the application of the approach in clinical studies.
Acknowledgments
The authors would like to thank the Carl Zeiss Surgical Group (Oberkochen Germany), for helpful information on the applicators’ characteristics used in the Intrabeam™ system.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frederik Wenz and Elena Sperk) for the series “Intraoperative Radiotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.02.01). The series “Intraoperative Radiotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin 2008;58:245-59. [PubMed]
- Sheehan JP, Jagannathan J. Review of spinal radiosurgery: a minimally invasive approach for the treatment of spinal and paraspinal metastases. Neurosurg Focus 2008;25:E18 [PubMed]
- Schneider F, Greineck F, Clausen S, et al. Development of a novel method for intraoperative radiotherapy during kyphoplasty for spinal metastases (Kypho-IORT). Int J Radiat Oncol Biol Phys 2011;81:1114-9. [PubMed]
- Yanch JC, Harte KJ. Monte Carlo simulation of a miniature, radiosurgery x-ray tube using the ITS 3.0 coupled electron-photon transport code. Med Phys 1996;23:1551-8. [PubMed]
- Bouzid D, Visvikis D, Dupré PF, et al. Dosimetric validation of an Intrabeam™ GATE model, based on Monte Carlo GEANT4 toolkit, for IORT applications. Med Phys 2013;40:SU-E-T-507, 322.
- Jan S, Benoit D, Becheva E, et al. GATE V6: a major enhancement of the GATE simulation platform enabling modeling of CT and radiotherapy. Phys Med Biol 2011;56:881-901. [PubMed]