ISIORT pooled analysis 2013 update: clinical and technical characteristics of intraoperative radiotherapy
Introduction
Intraoperative radiotherapy (IORT) is a special technique allowing the delivery of a single high radiation dose during the surgical procedure. IORT evolved as an attempt to improve the therapeutic ratio by achieving intensification of radiation dose while limiting irradiation of healthy structures which can be surgically displaced (1-3).
IORT can be combined with external beam radiation therapy (EBRT) or used as a single radiation dose to provide the best combination of loco-regional treatment. The first treatment of IORT was reported by Comas and Prio in 1905 in a case of endometrial cancer (4). Subsequently, other applications were described for other tumor locations, including abdomen, chest and head and neck by using low energy X-rays (5) and, in the 60ies, by using single high doses of gamma-rays of cobalt units and electrons of betatrons (6). The scenario changed in the early 90ies when dedicated mobile electron linear accelerators and a miniaturized low-energy X-rays machine were introduced into clinical practice. Looking at the articles published in those years, most papers on IORT focused on single institution series or retrospective reviews (7). More recently, retrospective series, large pooled analyses and also randomized trials were published with an increasing interest in integration of IORT into treatment of early-stage cancers, in particular breast cancer for boost or partial breast irradiation (PBI) settings.
In order to promote a scientific and professional approach to IORT activity, the International Society of Intraoperative Radiation Therapy (ISIORT) was founded in 1998 and the European section of ISIORT (ISIORT-Europe) was activated in 2006.
We recently published the first report on a data-base registry of the ISIORT-Europe with focus on clinical and technical aspects of IORT, in predominant tumor sites (8). This is an update including 2012/2013 data and 11 new centers.
Material and methods
Since 2007, the ISIORT-Europe centers were invited to record information of IORT treatments using the data-base registry. Real-time or retrospective data entry was allowed. Patient information was anonymized and integrated in a common data-base including demographic, clinical and technical information, tumor characteristic [such as staging according to TNM classification (9), treatment data, and specific IORT and EBRT data]. A detailed description of the data-base organization was published previously (8).
Results
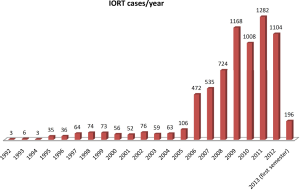
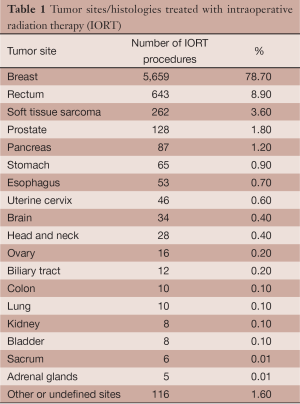
Thirty-one centers contributed to this survey recording data of 7,196 IORT procedures performed from 1992 to 2013. The number of centers increased from 3 in 2007, when the initiative was launched, to 21 in 2011 and to 31 most recently. The average number of patients treated per year in each center was 42 and exceeded 100 patients/year in 4 centers. The chronology of cumulative activity is described in Figure 1. Median age of patients was 60.6 years with range of 5 months-94 years. Gender was female in 80.2% of cases and male in 19.8%. Median performance status on Karnofsky scale was 90% with a range of 40-100%. Tumor types are reported in Table 1. Treatments were applied with megavoltage electron linear accelerators in 6,863 cases (95.4%) and with kilovoltage device in 333 cases (4.6%).
Full table
Treatments intent was curative in 7,054 cases (98%) and palliative in 142 cases (1.9%). One thousand five hundred and eighty seven cases (22.1%) were included in study protocols. Data from the seven most frequent tumor types are specifically analyzed and reported in details.
Breast cancer
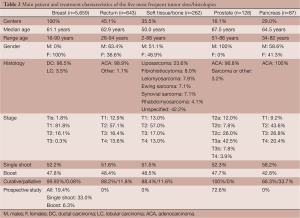
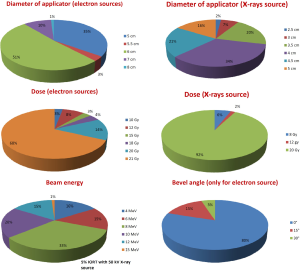
Data from 5,659 women with breast cancer were collected (Table 2). In 5,329 cases (94.1%), IORT was a component of radical treatment for primary newly diagnosed disease and in 330 cases (5.8%) it was an attempt to rescue localized recurrent breast cancer. In 52.2% of all indications, IORT was used as single radiation treatment modality with doses of 18 Gy (5%), 20 Gy (14.6%) or 21 Gy (80.4%) and in 47.8% as a boost before or after EBRT with doses of 8-12 Gy with electrons and from 8 to 20 Gy with low-energy X-rays. IORT was delivered before tumor removal in 37% of cases and after tumor removal in 63%. In case of recurrent tumor, 99% of IORT treatments were given as an exclusive radiotherapy component without EBRT. Patients enrolled in study protocols accounted for 20.2% of the whole series, mainly deriving from single-dose IORT trials (33.0% of all respectively treated patients) and only to a lesser extent from boost trials (6.3% of all boost-IORT patients). In 248 cases (5%), IORT was performed with a 50 kV energy X-rays source. In these cases, a single shot of 20 Gy was the most frequently used modality (92.5%). In 218 cases (87.9%), the treatment intent was curative. All these cases were included in a study protocol. As to clinical setting we did not observe apparent differences between the cohorts treated by electron and X-rays sources. Technical characteristics of treatments are described in Figure 2.
Full table
Rectal cancer
Six hundred and forty-three patients with rectal cancer were treated with IORT (Table 2). In 553 cases (86.0%), IORT was used for primary disease and in 90 cases (16.3%) for isolated local recurrence. In 81% of cases, IORT was part of a multidisciplinary approach including EBRT, chemotherapy and surgery. In 92.3% of cases, the surgeon obtained R0 resection. In 97% of cases, IORT was delivered after tumor removal. Some information about technical aspects: most used applicator was 6 cm diameter (33%), followed by 5 cm in 28% of cases; in 83% of cases bevel angle was 45°. Median dose to tumor bed was 10.8 Gy (range: 5-21.6 Gy), 33% of cases received 12.5 Gy and 27% of patients received 10 Gy with 12 MeV (33%) or 15 MeV (30%) beam energies, respectively. In 18 cases (3.9%), IORT was delivered by a 50 kV energy X-rays source, with spherical applicators of 3.5-5 cm in diameter, to total surface doses between 6 and 21.6 Gy with the majority of cases in palliative treatment intent (61.1%). In 4 cases (22.2%), IORT was the unique radiation modality.
Sarcoma
Data from 262 cases of sarcoma were available (Table 2). In 54.6% of cases, IORT was used for primary tumor and in 45.4% for local recurrence. In primary tumor setting, radical tumor excision was achieved in 84% of cases. A multimodal treatment comprising EBRT, IORT, chemotherapy, and surgery was performed in 19% of the cases. IORT was delivered after surgical resection in 99% of cases. A single field was used in 74% of cases, multiple fields in 15%, a field-within-field technique in 6% and adjacent fields in 5%. Large collimators with diameters of 12-15 cm were used in 26.5% of cases and small collimators in the range of 4-6 cm in 17.8% of cases. Bevel angle was 30° in 43% of cases and 15° in 26% of cases. Median dose amounted to 11.5 Gy (range, 5-25 Gy) and the most frequently administered doses were 10 Gy in 44% of the cases and 12.5 Gy in 36% of the cases. Radiation beam energies ranged from 6 to12 MeV. In 35 cases (13.4%), IORT was delivered with 50 kV X-rays with spherical applicators of 2.5-5 cm in diameter: 24 cases (68.6%) were recurrent tumors and the treatment intent was curative 29 cases (82.9%). Administered dose by X-rays was in the range of 5-20 Gy and in 20 cases (57.1%) the treatment was completed with EBRT.
Prostate cancer
One hundred twenty-eight patients with prostate cancer were treated with IORT in 5 centers (16.1%) (Table 2). All but 7 cases (94.5%) were primary, previously untreated tumors. IORT was used as a boost with doses of 8-15 Gy and as a single radiation modality with doses of 18-21 Gy. In 74.2% of cases, IORT was delivered prior to prostate removal. Diameters of applicator were between 4 and 6 cm. In 80% of cases, 30° bevel angles were used. The mostly used radiation beam energies were 9 MeV (43%) and 12 MeV (40%). In 6 cases (4.7%), IORT was delivered with a 50 kV X-rays source with spherical applicators of 5.6-8 cm in diameter. All these patients had recurrent tumors and the intent of treatment was palliative. In these cases, doses of 5-8 Gy in a single shot were administered.
Pancreatic cancer
Data from 87 patients treated with IORT in 9 centers (29%) for pancreatic cancer were available (Table 2). Three cases (3.7%) were recurrent tumors. In 22.8% of cases, IORT was applied to unresected tumors and in 73.6% of cases after pancreatectomy. The median dose delivered was 13.6 Gy (7.5-21 Gy). Diameters of applicator were between 6 and 9 cm. In 48% of cases, bevel angles were 15° or 30°. Radiation beam energies were uniformly distributed between 8 and 18 MeV. All cases were treated with an electron source.
Gastric cancer
Sixty-five patients with gastric cancer (adenocarcinoma, diffuse gastric cancer or gastric metastases) were treated with IORT in 4 centers (12.9%). Sixty-three patients were treated with curative intent, three of them (4.6%) affected by recurrent tumors, and two patients underwent surgery in palliative setting. In 59/65 cases (90.8%), surgeons obtained a radical resection.
Diameters of collimators ranged from 3 to 10 cm (8 cm in 32% of cases) and bevel angles from 0° to 30°, with 8 MeV as the most frequently used beam energy (47.7%). Administered doses were in the 7.5-15 Gy range and 15 Gy was the most used dose level. In two recurrent gastric tumors (3.1%), IORT was delivered with 50 kV X-rays with applicator of 5 cm in diameter and a single dose of 8 Gy.
Esophageal cancer
Two centers (6.6%) sent data about IORT in esophageal cancer comprising 51 primary and two recurrent tumors (adenocarcinoma or squamous cell carcinoma). In all cases, intraoperative irradiation was part of a multidisciplinary approach including EBRT, chemotherapy and surgery. In 15/51 patients (29.4%), surgery with IORT followed pre-operative EBRT. The administered dose was in the 7.5-15 Gy range with an energy between 6 and 12 MeV. All these cases were treated with electrons.
Discussion
This survey collected 7,196 IORT data from 31 worldwide institutions updating the results of a previous study on 3,754 treatments from 20 institutions (8). The increasing number of collaborating centers over time reflects a rising interest in this initiative.
The vast majority of treatments were performed with megavoltage electron linear accelerators (95.4%), and only a minority of 4.6% with orthovoltage devices.
The database shows that all contributing centers treated breast cancer with IORT. For patients with early-stage breast cancer, adjuvant breast cancer treatment using IORT offers the advantage of reducing overall treatment time and improving quality of patients’ life, as shown with the launch of large clinical trials exploring IORT (10-12), thus also reducing waiting lists in radiotherapy centers. Hence, increasing number of centers incorporate IORT in the multimodality treatment of breast cancer. The rationale of this treatment approach relies to the finding of about 85% of (at least first) local recurrences in the tissue adjacent to the lumpectomy site within five years of treatment after conservative treatment (13,14), prompting the interest for PBI to prevent majority of relapses. The efficacy of PBI by single shot IORT has been investigated in selected groups of patients (3,12,15,16). Prospective evidence about the potential of IORT as PBI strategy is generated mainly in two different multicenter trials: TARGIT and ELIOT, respectively, where 5-year experience is now available (17,18). With increasing follow-up periods, these studies will clarify if IORT-PBI following lumpectomy could be considered as a sole radiation option in selected patients with favorable prognostic factors as an alternative to postoperative EBRT.
In this regard, the European Society for Radiotherapy and Oncology (ESTRO) and the American Society for Radiation Oncology (ASTRO) formulated quite similar general criteria for the recommendation of PBI (19,20). However, only 33% of patients treated by a single radiation fraction were included in protocol studies, meaning that a large number of patients was treated considering high dose IORT as current clinical practice for selected patients with favorable prognostic features based on preliminary results of clinical trials: this issue is still matter of discussion in the scientific literature (21,22).The most frequently adopted dose level in our survey was 21 Gy as in the ELIOT trial (18). Single-fraction was also used as re-irradiation strategy after breast conserving for in-breast tumor in 5.8% of cases of our series.
IORT as a boost technique was used in 47.8% of the patients and mostly outside clinical trials meaning that the approach of anticipated boost is adopted as a current practice. In expert IORT institutions, dose intensification has proven to obtain outstandingly low rates of local recurrence in already reported clinical studies (23,24).
IORT in rectal cancer aims to improve local control in locally advanced high-risk disease and in recurrent tumors where pelvic relapse is responsible for therapeutic failure. Undisputedly, achievement of R0 resection is the most important prognostic factor in colorectal cancer for subsequent local control. In situations where the radial margin is close or on vessels that are not amenable to resection, the goal of IORT is to eradicate microscopic tumor cells remaining within a few millimeters of the final surgical margin, while at the same time avoiding radiation exposure to small bowel, ureters, and bladder. Most cases of our analysis presented with locally advanced stage and a non-negligible percentage of patients (16.3%) were treated for locally recurrent disease. In the large majority of cases, IORT was given with curative intent as boost intensification dose and was part of a multidisciplinary approach including surgery, EBRT, and chemotherapy.
Several literature studies showed a favorable local effect of IORT with high rates of local control in advanced primary and in recurrent rectal tumors (25-31). Unfortunately, at the moment, there is only one phase III randomized trial compared preoperative radiotherapy followed by surgery (standard arm) with surgery and IORT (experimental arm) in 142 patients with a clinically T3, T4 or N+ and M0 rectal cancer (32). This trial failed to show an advantage for the experimental arm. A collective effort in designing and enrolling patients in prospective clinical trials may generate evidence in tailoring the indication of IORT for rectal cancer (33). Technical parameters of our survey were adequate to treat the presacral space and were quite similar to those reported in the European pooled analysis (34). Further developments in rectal cancer include the integration of IORT presacral electron boost during laparoscopic radical surgery (35).
IORT is used in the multimodality treatment of sarcoma because it enables the application of high-dose radiation to target volume and makes possible a lower EBRT dose with corresponding inferior dose to surrounding healthy tissues. IORT could also better avoid radiation exposure to joint spaces in extremities sarcoma. There is a large amount of published work on the use of IORT, and also HDR-IORT, in combination with surgery with or without EBRT for the treatment of retroperitoneal soft-tissue sarcoma and in sarcoma localized in the trunk or in the extremities (36-40). However, these studies are heterogeneous because in all of them there were patients who received IORT alone, while others received IORT plus EBRT, with the latter pre- or postoperatively. Prescription points and modalities also vary between the studies. Similarly our analysis showed quite heterogeneous subtypes. Almost half of the patients had recurrent tumors. In terms of technical aspects, soft tissue sarcoma required a wide range of applicator diameters and bevel angles, most likely in relation with the frequently large tumor extension and the post-resection tumor bed in soft tissues. Moreover, many cases required complex irradiations with multiple fields, or field within a field with high energy electrons up to 18 MeV and doses up to 25 Gy. Similar treatment modalities were described in other literature reports (37-39,41).
The rationale for dose escalation with IORT for prostate cancer is based on the demonstration of dose-response relationship and on the low α/β value in the linear quadratic model, a concept increasingly investigated in hypofractionated EBRT (42,43). Italian authors reported data using IORT in combination with radical prostatectomy and regional lymph node dissection before or after the surgical procedure (44-47). A large part of these patients (72.6%) was included in prospective institutional study protocols. In most of the cases, IORT was used as a boost dose prior to prostate removal with doses of 8-15 Gy and as a single radiation modality with doses of 18-21 Gy for single shot IORT. In our survey, a dose of 18-21 Gy was adopted. The diameter and the bevel angle end of applicators were chosen on the basis of the target dimension considering a margin of at least 5 mm around the prostate and the necessity to reach the target underneath the pubic arch by sparing the bladder.
IORT is a potentially advantageous therapeutic approach for dose intensification to improve local control in locally advanced pancreatic cancer that still represents one of the most lethal malignancies in males with an overall survival of less than 5% at five years. From literature, benefit of the addition of IORT in patients was described for localized disease in particular in two multicentre analyses (48,49).
The registry has collected patients with locally advanced stages (mainly T2-T4) of pancreatic tumors, treated in most of the cases with curative intent after tumor resection. Most of these cases received multimodality treatments (surgery, adjuvant EBRT, neoadjuvant/adjuvant chemotherapy, biological targeted therapy). Case selection corresponded quite well to that of other literature studies (49). From the technical point of view, quite large diameters of applicators were used with a large range of beam energies and dose levels, most likely depending on the respective need to irradiate the unresected pancreas or the surgical tumor bed plus/minus regional lymph nodes. Recent data has reported that, in the context of chemoradiation and resection, the addition of IOERT boost significantly improves loco-regional control (50).
Defining the optimal role of radiotherapy regimens in the treatment of locally advanced gastric and esophageal malignancies remains an elusive goal. Although surgery remains the mainstay of curative therapy for both esophageal and gastric malignancies, investigators have long noted a high risk of local and regional recurrence in these malignancies. In this context, IORT has been investigated since Abe’s first experience (6).
Concerning gastric cancer, an increase in loco-regional control of 12-15% has been reported in patients with IORT compared with those who did not receive IORT. However, overall survival has not improved in these series and complication rates are significantly higher following the use of IORT, which should be weighed carefully against the potential benefits (7,51-53). These elements explain why in the present survey only few and highly specialized centers have treated gastric cancer with IORT. From the technical point of view, a large diameter collimator was needed in most of the cases for an adequate coverage of the surgical bed and lymphatic drainages.
As far as esophageal cancer is concerned, IORT can be a convenient modality to treat the lymph nodal volume which is a critical issue in particular in lesions located at the organ’s middle and lower third. According to several studies, 58-74% of patients undergoing esophagectomy for thoracic esophageal carcinoma were diagnosed histologically as having lymph nodes metastasis (54). Recently, a study was published about the role of IORT targeted to the abdominal lymph node area in patients with esophageal carcinoma. This study revealed that the survival rate was significantly higher in favor for the use of IORT, without perioperative complications solely attributable to intraoperative irradiation (55). Of note in the present survey, all patients were treated in the context of a multidisciplinary approach meaning that the IORT boost was considered as a potentially advantageous modality for local dose intensification. In this context, a contemporary single institution cohorts analysis has shown that IORT electron boost, in post-chemoradiation resected status of locally advanced esophageal and gastro-esophageal junction carcinoma, significantly improves loco-regional control but not survival (56).
Oligo-recurrent and oligometastatic cancer are new clinical entities in which intensive local therapy (including radiation containing strategies) have proven to induce long-term survivors not expected with more conventional or conservative approaches (57). Systematic reviews have identified outcome benefits for the use of IORT components in the rescue of locally recurrent rectal cancer (58). Long-term single institution data have confirmed the potential of IORT in oligometastatic extrapelvic cancer (59) and oligo-recurrent gynecological cancer models (60).
The data presented are an update of the first report on a large clinical experience on patients treated with IORT and gives an overview on practice oriented patients’ selections. Moreover, this survey describes aspects of patients’ selection, chronological activity, treatment strategies and technical modalities for a number of tumor types which are currently treated and may benefit from this technique. The increasing number of collaborating centers will allow performing further data analyses based on a larger number of treatments, which could represent a solid basis for future collaborative prospective trials by identification of clinical partners.
Acknowledgments
The authors thank the following colleagues for sending data of their Centers:
Morena Sallabanda, University Hospital Gregorio Maranon, Madrid, Spain; Bernhard Mitterlechner, University Hospital of the Paracelsus Medical University, Salzburg, Austria; Franco Checcaglini and Fabrizio Fusconi, Hospital, Città di Castello, Italy; Sergio Maluta, Hospital, Verona, Italy; Renzo Corvò, University Hospital and Cancer Centre, Genova, Italy; Sebastian Adamczyk, Greater Poland Cancer Centre, Poznan, Poland; Elvio Russi and Claudia Fillini, Hospital Santa Croce e Carle, Cuneo, Italy; Fabrizio Fusconi, Hospital San Giovanni Battista, Foligno, Italy; Riccardo Maurizi Enrici and Mattia Osti, University Hospital Sant’Andrea, Rome, Italy; Luigi Tomio, Hospital Santa Chiara, Trento, Italy; Hugo Marsiglia and Ignazio Azinovic, Instituto Madrileño de Oncología, Madrid, Spain; Antonella Ciabattoni, Hospital San Filippo Neri, Rome, Italy; Wojciech Polkowski, UniversityHospital, Lublin, Poland; Alfio Di Grazia, IOM Catania, Italy; Alessandro Gava Hospital, Treviso, Italy; Abraham Kuten, Rambam Health Care Campus, Haifa, Israel; Cinzia Iotti, Hospital Santa Maria Nuova, Reggio Emilia, Italy; Jean-Bernard Dubois, Centre Régional de Lutte contre le Cancer Val d’Aurelle Montpellier, France; Gianpiero Catalano, Hospital Multimedica, Castellanza, Italy; Franco Cazzaniga, Ospedale San Giovanni XXIII, Bergamo, Italy; Claudia Schumacher, St. Elisabeth-Krankenhaus, Koln, Germany; Reinhilde Weytjens, Sint Augustinus Hospital Wilrijk, Belgium; Bellaria, Antonella Baldissera, Hospital Bellaria, Bologna, Italy; Carlos Ferrer, Virginia Morillo, Juan Lopez-Tarjuelo Hospital Provincial of Castellon, Spain; Francesco Richetti, Hospital Sacro Cuore, Negrar, Italy; Vincenzo Fusco, IRCCS-CROB, Rionero in Vulture, Italy; Leonardo Badinez, Fundacion Arturo Lopez Perez, Santiago de Chile, Chile.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Intraoperative Radiotherapy”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.01.02). The series “Intraoperative Radiotherapy” was commissioned by the editorial office without any funding or sponsorship. FW served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. ES served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki. The institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sindelar WF, Kinsella TJ. Normal tissue tolerance to intraoperative radiotherapy. Surg Oncol Clin N Am 2003;12:925-42. [PubMed]
- Willich N. Technical and methodical developments of radiation oncology from a physician’s point of view. Strahlenther Onkol 2012;188:253-62. [PubMed]
- Wareńczak-Florczak Z, Roszak A, Bratos K, et al. Intraoperative radiation therapy as part of breast conserving therapy of early breast cancer—results of one-year follow-up. Rep Pract Oncol Radiother 2013;18:107-11. [PubMed]
- Comas C, Prio A. Irradiation roentgen preventive intraabdominale, après l’intervention chirurgicale dans un cas de cancer de l’uterus. Pesented at the Congres International d’Electrologie, Imprenta Francesca Badia, Barcelona 1906.
- Gunderson LL, Willet CG, Calvo FA, et al. Intraoperative irradiation techniques and results, 2nd ed. Current clinical oncology. New York: Humana Press, 2011.
- Abe M, Takahashi M. Intraoperative radiotherapy: the Japanese experience. Int J Radiat Oncol Biol Phys 1981;7:863-8. [PubMed]
- Debenham BJ, Hu KS, Harrison LB. Present status and future directions of intraoperative Radiotherapy. Lancet Oncol 2013;14:e457-64. [PubMed]
- Krengli M, Calvo FA, Sedlmayer F, et al. Clinical and technical characteristics of intraoperative radiotherapy: analysis of the ISIORT-Europe database. Strahlenther Onkol 2013;189:729-37. [PubMed]
UICC-TNM Classification of Malignant Tumours 2009 .- Veronesi U, Orecchia R, Luini A, et al. Intraoperative radiotherapy during breast conserving surgery: a study on 1,822 cases treated with electrons. Breast Cancer Res Treat 2010;124:141-51. [PubMed]
- Williams NR, Pigott KH, Keshtgar MRS. Intraoperative radiotherapy in the treatment of breast cancer: a review of the evidence. Int J Breast Cancer 2011;2011:375170.
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [PubMed]
- Vaidya JS, Vyas JJ, Chinoy RF, et al. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer 1996;74:820-4. [PubMed]
- Baum M, Vaidya JS, Mittra I. Multicentricity and recurrence of breast cancer. Lancet 1997;349:208. [PubMed]
- Valachis A, Mauri D, Polyzos NP, et al. Partial breast irradiation or whole breast radiotherapy for early breast cancer: a meta-analysis of randomized controlled trials. Breast J 2010;16:245-51. [PubMed]
- Sperk E, Welzel G, Keller A, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253-60. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [PubMed]
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). J Am Coll Surg 2009;209:269-77. [PubMed]
- Polgár C, Van Limbergen E, Pötter R, et al. GEC-ESTRO breast cancer working group patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010;94:264-73. [PubMed]
- Sautter-Bihl ML, Sedlmayer F, Budach W, et al. Intraoperative radiotherapy as accelerated partial breast irradiation for early breast cancer: beware of one-stop shops? Strahlenther Onkol 2010;186:651-7. [PubMed]
- Sedlmayer F, Sautter-Bihl ML, Budach W, et al. DEGRO practical guidelines: radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol 2013;189:825-33. [PubMed]
- Sedlmayer F, Fastner G, Merz F, et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: results of an ISIORT Pooled analysis. Strahlenther Onkol 2007;183:32-4. [PubMed]
- Fastner G, Sedlmayer F, Merz F, et al. IORT with electrons as boost strategy during breast conserving therapy in limited stage breast cancer: long term results of an ISIORT pooled analysis. Radiother Oncol 2013;108:279-86. [PubMed]
- Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for primarly locally advanced rectal and rectosigmoid carcinoma. J Clin Oncol 1991;9:843-9. [PubMed]
- Calvo FA, Gómez-Espí M, Díaz-González JA, et al. Intraoperative presacral electron boost following preoperative chemoradiation in T3-4Nx rectal cancer: initial local effects and clinical outcome analysis. Radiother Oncol 2002;62:201-6. [PubMed]
- Krempien R, Roeder F, Oertel S, et al. Long term results of intraoperative presacral electron boost radiotherapy (IOERT) in combination with total mesorectal excision (TME) and chemotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2006;66:1143-51. [PubMed]
- Mathis KL, Nelson H, Pemberton JH, et al. Unresectable colorectal cancer can be cured with multimodality therapy. Ann Surg 2008;248:592-8. [PubMed]
- Haddock MG, Miller CR, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 2011;79:143-50. [PubMed]
- Yeo HL, Pati PB. Management of recurrent rectal cancer: practical insights in planning and surgical intervention. J Surg Oncol 2014;109:47-52. [PubMed]
- Calvo FA, Sole CV, Alvarez de Sierra P, et al. Prognostic impact of external beam radiation therapy in patients treated with and without extended surgery and intraoperative electrons for locally recurrent rectal cancer: 16-year experience in a single institution. Int J Radiat Oncol Biol Phys 2013;86:892-900. [PubMed]
- Dubois JB, Bussieres E, Richaud P, et al. Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study. Radiother Oncol 2011;98:298-303. [PubMed]
- Valentini V, Schmoll HJ, van de Velde CJH. Multidisciplinary management of rectal cancer. Springer, 2012.
- Kusters M, Valentini V, Calvo FA, et al. Results of European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol 2010;21:1279-84. [PubMed]
- Calvo FA, Sole CV, Serrano J, et al. Postchemoradiation laparoscopic resection and intraoperative electro-beam radiation boost in locally advanced rectal cancer: long-term outcomes. J Cancer Res Clin Oncol 2013;139:1825-33. [PubMed]
- Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg 1993;128:402-10. [PubMed]
- Petersen IA, Haddock MG, Donohue JH, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys 2002;52:469-75. [PubMed]
- Azinovic I, Martinez Monge R, Javier Aristu J, et al. Intraoperative radiotherapy electron boost followed by moderate doses of external beam radiotherapy in resected soft-tissue sarcoma of the extremities. Radiother Oncol 2003;67:331-7. [PubMed]
- Krempien R, Roeder F, Oertel S, et al. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 2006;65:773-9. [PubMed]
- Oertel S, Treiber M, Zahlten-Hinguranage A, et al. Intraoperative electron boost radiation followed by moderate doses of external beam radiotherapy in limb-sparing treatment of patients with extremity soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 2006;64:1416-23. [PubMed]
- Calvo FA, Sole CV, Cambeiro M, et al. Prognostic value of external-beam radiation therapy in patients treated with surgical resection and intraoperative electron-beam radiation therapy for locally recurrent soft tissue sarcoma: a multicentric long-term outcome analysis. Int J Radiat Oncol Biol Phys 2014;88:143-50. [PubMed]
- Alongi F, Fogliata A, Navarria P, et al. Moderate hypofractionation and simultaneous integrated boost with volumetric modulated arc therapy (RapidArc) for prostate cancer. Report of feasibility and acute toxicity. Strahlenther Onkol 2012;188:990-6. [PubMed]
- Geier M, Astner ST, Duma MN, et al. Dose-escalated simultaneous integrated-boost treatment of prostate cancer patients via helical tomotherapy. Strahlenther Onkol 2012;188:410-6. [PubMed]
- Saracino B, Gallucci M, De Carli P, et al. G. Phase I-II study of intraoperative radiation therapy (IORT) after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys 2008;71:1049-56. [PubMed]
- Rocco B, Jereczek-Fossa BA, Matei DV, et al. Intraoperative radiotherapy during radical prostatectomy for intermediate-risk to locally advanced prostate cancer: treatment technique and evaluation of perioperative and functional outcome vs standard radical prostatectomy, in a matched-pair analysis. BJU Int 2009;104:1624-30. [PubMed]
- Krengli M, Terrone C, Ballarè A, et al. Intraoperative radiotherapy during radical prostectomy for locally advanced prostate cancer: technical and dosimetric aspects. Int J Radiat Oncol Biol Phys 2010;76:1073-7. [PubMed]
- Krengli M, Terrone C, Jereczek-Fossa BA, et al. May intra-operative radiotherapy have a role in the treatment of prostate cancer? Crit Rev Oncol Hematol 2012;83:123-9. [PubMed]
- Ruano-Ravina A, Almazán Ortega R, Guedea F. Intraoperative radiotherapy in pancreatic cancer: a systematic review. Radiother Oncol 2008;87:318-25. [PubMed]
- Valentini V, Calvo F, Reni M, et al. Intra-operative radiotherapy (IORT) in pancreatic cancer: Joint analysis of the ISIORT-Europe experience. Radiother Oncol 2009;91:54-9. [PubMed]
- Calvo FA, Sole CV, Atahualpa F, et al. Chemoradiation for resected pancreatic adenocarcinoma with or without intraoperative radiation therapy boost: long-term outcomes. Pancreatology 2013;13:576-82. [PubMed]
- Calvo FA, Sole CV, Obregón R, et al. Intraoperative radiotherapy for the treatment of resectable locally advanced gastric adenocarcinoma: topography of locoregional recurrences and long-term outcomes. Clin Transl Oncol 2013;15:443-9. [PubMed]
- Zhang Q, Tey J, Peng L, et al. Adjuvant chemoradiotherapy with or without intraoperative radiotherapy for the treatment of resectable locally advanced gastric adenocarcinoma. Radiother Oncol 2012;102:51-5. [PubMed]
- Fu S, Lu JJ, Zhang Q, et al. Intraoperative radiotherapy combined with adjuvant chemoradiotherapy for locally advanced gastric adenocarcinoma. Int J Radiat Oncol Biol Phys 2008;72:1488-94. [PubMed]
- Ide H, Nakamura T, Hayashi K, et al. Esophageal squamous cell carcinoma: pathology and prognosis. World J Surg 1994;18:321-30. [PubMed]
- Tamaki Y, Sasaki R, Ejima Y, et al. Efficacy of intraoperative radiotherapy targeted to the abdominal lymph node area in patients with esophageal carcinoma. J Radiat Res 2012;53:882-91. [PubMed]
- Calvo FA, Sole CV, Obregón R, et al. Postchemoradiation resected locally advanced esophageal and gastroesophageal junction carcinoma: long–term outcome with or without intraoperative radiotherapy. Ann Surg Oncol 2013;20:1962-9. [PubMed]
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [PubMed]
- Mirnezami R, Chang GJ, Das P, et al. Intraoperative radiotherapy in colorectal cancer: systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg Oncol 2013;22:22-35. [PubMed]
- Calvo FA, González ME, González-San Segundo C, et al. Surgery and intraoperative electron radiotherapy in recurrent or metastatic oligotopic extrapelvic cancer: Long-term outcome. Eur J Surg Oncol 2012;38:955-61. [PubMed]
- Calvo FA, Sole CV, Lozano MA, et al. Intraoperative electron beam radiotherapy and extended surgical recection for gynaecological pelvic recurrent malignancies with and without external beam radiation therapy: long-term outcomes. Gynecol Oncol 2013;130:537-44. [PubMed]