
Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects
Introduction
Ionizing radiation (IR) is used as a main treatment for many types of localized solid tumors where radiation therapy (RT) is considered the primary non-surgical modality in the curative treatment of cancer (1,2). Although chemotherapy (CT) and traditional fractionated radiation have been described as immunosuppressive (2), recent data suggests that RT can modulate anti-tumor immune responses (3), modifying tumor and its microenvironment (4).
Radiotherapy and the immune system modulation
Besides the direct effects of radiation in reducing viable cancer cells, RT may induce modifications on the local microenvironment that can affect tumor development (5). Most tumor cells do not express major histocompatibility complex (MHC) class II. As a consequence, they cannot directly activate the specific CD4+ T cell-mediated tumor immunity, which is essential for the development of adaptive immune responses. Tumor cells develop multiple and complex mechanisms to fully escape immune surveillance. These cells can produce immunosuppressive cytokines and the recruitment of inhibitory and regulatory cell types, decrease expression of antigens, lose expression of MHC class I molecules, have an aberrant antigen processing, cause anergy or deletion of T cells, and generate the dysfunction of dendritic cells (DCs) (5-8). The interaction of all these factors could lead to cancer cells escaping the immune system (6).
It has been shown that RT may contribute to making tumors visible to the immune system (9-14). After RT treatment, there is an increase pool of peptides for antigen presentation displayed by MHC-I molecules (6). The tumor-associated derived antigens (TAAs) released to the tumor periphery can be captured by DCs. These DCs become active via toll-like receptors (TLRs) recognition, in which endogenous danger signals emitted by dying tumor cells are identified. The activation of DCs is characterized by the upregulation of cell surface molecules involved in antigen presentation and costimulation (e.g., CD80, CD86) and the release of pro-inflammatory cytokines. Thus, activated DCs migrate to secondary lymphoid organs where TAAs will be presented to CD4+ Th cells in the context of MHC-II. Active, effector, T cells may recirculate through the body and generate a tumor-specific immune response in distant areas. By means of this mechanism, adaptive immune responses may help to eradicate metastasis of tumors that do not express MHC-II. CD4+ T cells may help to kill tumor cells by several mechanisms. One such is enabling the development of tumor specific CD8+ T cells which recognize tumor peptides by MHC-I (Figure 1). CD4+ T cells, particularly Th1 cells, secrete interferon (IFN)-γ, which induces MHC-I expression in tumor cells. IFN-γ may also collaborate to control tumor growth by inhibiting angiogenesis. In addition, tumors treated with RT increase the IFN-γ-production, which in turn upregulates MHC-I expression. Despite the central role of CD4+ T cells on anti-tumor adaptive immunity, exogenous antigens such as TAAs, may be presented by DCs via cross-priming to CD8+ T cells in the context of MHC-I; this process could take place without a previous CD4+ T cell helping. This would support the hypothesis that radiation may enhance the tumor immunogenicity by promoting cross-priming and stimulating the effector phase of the anti-tumor immune response (1,10,15). In addition, RT can induce the secretion of a wide range of cytokines and other mediators by RT-targeted tumor cells and surrounding cells (such as endothelial cells of tumor stroma and infiltrating tumor cells).
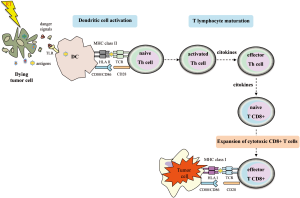
Tumor cells and tumor microenvironment
The factors involved in non-targeted effects are likely to be multiple, and include cell-to-cell gap junctions, reactive oxygen species (ROS), reactive nitrogen species [e.g., nitric oxide synthase (iNOS)], cytokines and chemokines (16).
It is well known that IR has direct effects on DNA damage altering the phenotype of tumor cells (targeted effects) (4,17). Besides this, the effects of RT may also be detected in non-irradiated cells that are in the vicinity of irradiated cells. This phenomenon is called “bystander effect”, and it has been observed in a wide range of cell types and for several biological end points (DNA damage, genomic instability, oncogenic transformation and cell death) (18,19). Multiple studies have shown that IR also induces an effect on cells that are at a distance from the primary tumor. Since the 1950’s, it had been observed that tissues that were outside the irradiation area, responded as if they were being irradiated, however the cause was unknown. Over the last decade, it has been postulated that the effects induced on tumors treated with local irradiation are immune-mediated, and T-cells are required for distant effects. The effect in non-irradiated tissues located outside the radiation field is termed “abscopal effect” (3,9,15,17,20,21).
Non-targeted effects: local (bystander effects)
Radiation-induced bystander effect is a universal mode of intercellular communication and distant cell signaling that is not restricted to radiobiological processes (20). This phenomenon has been observed in numerous cell types (e.g., lymphocytes, endothelial cells, fibroblasts and tumor cells) and tissue models, as well as in vivo (animal models). These non-targeted effects in non-irradiated cells are mediated via cell-to-cell gap junctions and through mediators released from irradiated cells, especially cytokines and chemokines (22,23). At cellular levels, bystander effects include genomic instability and signaling effects that can lead to either cell activation or cell death, particularly by apoptosis (20,24). A common hallmark of bystander effects is that there is no clear dose-response relationship. The clinical response to RT improves with increased radiation doses, but reaches a plateau at relatively low doses (2,23,25). Bystander effects predominate with low-to-moderate doses of radiation, and little to no further increase is observed at higher doses of radiation (2). Moreover, it has been suggested that epigenetic changes mediated by microRNAs may act in the variability of bystander responses (19).
During the stress response induced by localized radiation, the cellular effects induced by this phenomenon can contribute to a type of cell death that is immunogenic, and involves changes in the cell surface composition and release soluble immunogenic signals to initiate an effective immune response (5,26). These non-targeted effects could be considered as the whole immunological response of tumor and normal tissues to RT-induced stress. Additionally, bystander effects have been documented in response to non-IR and CT, supporting the concept that it is a stress-related and generalized response strategy (2). Despite the fact that the mechanisms behind this phenomenon are still under discussion, it is considered that oxidative and inflammatory response may play a central role (27). More evidences suggest that bystander effects induced by radiation are, at least in part, immune mediated (19). Although bystander responses become dominant at low-to-moderate doses, it could have a significant role even after high doses are applied. A study from Fernandez-Palomo et al. (28), provided data about the presence of bystander effects in rats after high radiosurgical doses of synchrotron radiation. The authors suggested a difference between the bystander effects produced in tumor free-tissue and the tumor, the latter effect being higher. It is conceivable that bystander routes in vivo could be more complex than in cell cultures (28). Therefore, in vivo models allow a better way to represent non-targeted effects as the whole immunological mechanism of tumor and normal tissue, which could include both bystander and abscopal effects (2,28).
Some of the key pathways and mechanisms implicated in the bystander response are still being elucidated. Bystander effects have been found either in tumor and normal cells, but not all cell types produce bystander signals and respond in the same way. In addition, cancer cell killing induced by radiation does not distinguish between cells more susceptible to the immune system versus cells that are indeed more resistant (6,15,29). Some biomarkers such as inflammatory factors, genomic instability, ROS and cytokines might be related to the bystander effects. More information is needed to identify the mediators and mechanisms implicated in the bystander effects. In addition it was suggested that the effect of some of these mediators may be beneficial or harmful for tumor development (29).
Microenvironment
RT modifies the phenotype of tumor cells, but it also has a significant impact in the local microenvironment. Non-targeted effects generated in response to radiation exposure are mediated by immune signaling-related mechanisms, affecting surrounding non-irradiated cells (4,5). In most of cancers, both in the tumor and its microenvironment, there is a balance between immune cells that mediate tissue destruction and immune cells that work to prevent that destruction (30). In addition to neoplastic cells, the microenvironment of solid tumors modified by radiation results in an increased vascular permeability, local inflammation, and altered cytokine production. Tumor-associated macrophages (TAMs) play a central role in the connection between inflammation and cancer. TAMs exert a variety of functions, including tumor progression, angiogenesis, matrix deposition, production of immunosuppressive cytokines, and repression of adaptive immunity which ultimately have an important impact on disease progression (5,30).
Cytokines and chemokines
It has been demonstrated that the immune system is an active participant in cancer initiation, progression and pathogenesis, exerting both pro- and anti-tumorigenic activities (5). RT has a significant effect on the modulation of immune responses, and this effect is largely due to the production of cytokines and chemokines both by the tumor cells and by the tumor stroma (13,22). Cytokines can be either immune stimulating, pro-inflammatory, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, or IL-1, or immune suppressive, anti-inflammatory (e.g., IL-10).
Many cell types of the immune system produce cytokines and chemokines, including macrophages, lymphocytes and granulocytes. Nevertheless, other cells, not necessarily from the immune system, such as endothelial cells and fibroblasts, may also produce a vast array of cytokines and chemokines. Some cytokines and chemokines can not only display anti-tumor direct effects but can also influence chemotaxis and tumor infiltration by leukocytes, as well as suppress or stimulate the immune system activity. In addition several cytokines and chemokines can regulate the neovascularization process by inducing [e.g., monocyte chemoattractant protein-1 (MCP-1) and IL-8] or inhibiting angiogenesis [e.g., IFN-γ-inducible protein 10 (IP-10) and monokine-induced by IFN-γ (MIG)] (31). In any event, depending on several factors (e.g., cytokine concentrations, presence of other modulating factors, microenvironment, and stages of cancer progression), a particular cytokine or chemokine may stimulate different responses and may mediate both acute and late tissue responses to IR (32,33). These molecules are able to act outside the tumor burden as well as systemic level (17,34,35).
IFN-γ, mainly produced by T helper (Th) 1, natural killer (NK), and natural killer T (NKT) cells is a potent pro-inflammatory cytokine, critical in tumor immunity). IFN-γ acts on macrophages and helps to eliminate pathogens. Initially, the IFN-γ amount released on the tumor area induces local chemokines production, which helps to recruit more cells of the innate immune system in the tumor. After RT, IFN-γ produced within the tumor microenvironment, also leads to infiltration of T-cells (9). IFN-γ enhances the cytotoxicity of macrophages and the maturation of DCs. It is thought that under radiation conditions, several IFN-γ-dependent mechanisms could enhance cytotoxic T lymphocytes (CTLs) trafficking, which facilitates the recognition of tumor cells through the upregulation of antigen presentation and MHC-I expression. The key role of IFN-γ in the anti-tumor response is highlighted by the fact that one of the mechanisms that allow tumors to escape from elimination by the immune response is the development of IFN-γ insensitivity. In fact, some human tumors naturally develop mutations in genes coding for IFN-γ signaling proteins as a mechanism to evade immunosurveillance (8,31,36-38). The IL-12/IFN-γ axis has been extensively reported to be implicated in tumor surveillance mechanisms. In fact, inborn errors of the IL-12/IFN-γ circuit may also predispose to both viral and non-viral-induced cancers in mouse models (39). Different strains of IFN-γ deficient mice differ in their susceptibility to spontaneous tumor development, and mutations in p53 increase the spectrum of tumors observed in IFN-γ insensitive mice (8,40). In the last years, several patients with deficiencies in components of the IL-12-IL-23/IFN-γ circuit where reported to suffer from cancers at young ages. These results are reminiscent of those observed in mice models, and suggest that patients with deficiencies of the IL-12-IL-23/IFN-γ axis seem to be more susceptible to cancer, particularly to viral-induced tumors (8,36-38). IFN-γ has pleiotropic effects in the tumor microenvironment. It can induce the expression of surface MHC-I molecules, activate macrophages, inhibit the production of immunosuppressive molecules, and enhance the secretion of antiangiogenic chemokines. After tumor irradiation, there is an increase in IFN-γ-production. However, for an effective anti-tumor immunity, it is necessary to balance the positive and negative effects of IFN-γ (14). IL-12 produced by macrophages and DCs in response to bacterial stimuli, act on T, NK, and NKT cells to induce IFN-γ production. Dependent on the cytokine milieu, effector T cells differentiate into Th1, Th2 and Th17 (41). IL-23 promotes the expansion of IL-17-polarized Th cells. However IL-23 may exert a dual role in cancer: the administration of excessive amounts of IL-23 was associated with significant anti-tumor immune responses in tumor mouse models, whereas endogenous IL-23 was reported to promote tumor incidence and growth in vivo. The latter effect was proposed to occur through pro-inflammatory and proangiogenic effector pathways that sustain the tumor, which may be mediated by IL-17 (36,42). Expression of IL-23, but not of IL-12, is increased in human tumors, and both cytokines antagonistically regulate local inflammatory responses in the tumor microenvironment. In addition, IFN-γ-independent anti-tumor activities of IL-12 and IL-23 have also been reported, e.g., IL-12 has shown to reduce DNA lesions induced by ultraviolet (UV) (36).
Some studies emphasize the elevated basal expression of transforming growth factor beta (TGF-β), IL-1β and IL-8 in diverse tumor types (13). TNF-α and IL-1β initiate the immune system activity, whereas other cytokines such as TGF-β, IL-10 and IL-4 may suppress the immune response (21). The aim of RT is to obtain a maximized tumor control with the minimum injury of normal tissues. TGF-β participates in the homeostatic growth control, but it has a more complex role in regulating tissue responses to damage. TGF-β may play a dual role both limiting tumor growth and stimulating tumor cells progression (13,43). It seems that in the early stages of cancer progression, TGF-β acts as an antitumorigenic factor, but at later stages it becomes protumorigenic (20). In some circumstances, TGF-β activation elicited by IR may promote tumor growth rather than to delay it (43). Several studies in rodents suggest that limiting TGF-β signaling during radiotherapy might decrease damage in normal tissues (40). Moreover, the secretion of TGF-β may be inhibited by IFN-γ. Several cancer cells, infiltrating fibroblasts, DCs and tumor-infiltrating lymphocytes (TILs), can produce TGF-β. The balance between TGF-β and IL-6 plays an important role in the development of Th17 and regulatory T (Treg) cells. Treg cells are a distinct T cell subpopulation that is involved in mediating immunological self-tolerance and homeostasis (44). An overactive Treg cell function may contribute to the suppression of tumor immunity. In an anti-inflammatory milieu, with low or absence of IL-6 levels, TGF-β would promote the expansion of Treg population against Th17 cells (45). Myeloid-derived suppressor cells (MDSCs) are a population of immature myeloid cells and a source of TGF-β production, which promotes immune tolerance and contribute to angiogenesis and vasculogenesis, stimulating tumor invasion and metastasis (5,21).
On the other hand, TNF-α is a cytokine secreted during the initial phase of tumor response, and it has a strong inflammatory and pro-apoptotic activity (3,13). Many cell types may produce TNF-α molecules. This cytokine can inhibit tumor angiogenesis working together with RT. However, as TGF-β cytokine, TNF-α may have dual effects on tumor development: at low concentrations, TNF-α promotes tumor angiogenesis, tumor cell survival and metastasis, but at high levels it could have anti-tumorigenic effects (21).
The expression of certain cytokines, such as TNF-α, IL-1 and IL-6 has been shown to be increased after irradiation, and may be involved in non-targeted effects. In such a case, inflammation combined with RT could be beneficial (3). IL-6 is a pro-inflammatory cytokine, and it has been implicated in several types of cancer. It seems to be related with tumor grade and stage. In fact, IL-6 is an effector signal that activates nuclear factor (NF)-κB which is critical for cancer progression. It has been shown that IL-6 produced by the primary tumor, can act as a growth factor in the primary tumor, and also at distant metastatic sites (9,46).
IL-1β is an additional pro-inflammatory cytokine that induces proliferation or apoptosis depending on stimulus type and target cell stage. A natural competitive inhibitor of IL-1, the IL-1 receptor antagonist (IL-1ra), may regulate the inflammatory response by blocking the IL-1 receptor activation (13).
IL-10, an anti-inflammatory cytokine, is involved in the suppression of immune responses. Different tumor cells, including gastric carcinoma, melanoma and squamous cell carcinoma and other cells that take part in tumor microenvironment are an important source of IL-10 (21).
As discussed above, RT can modulate the cytokine production by tumor cells (20). Yamamoto et al. (47). observed that after irradiation, TILs had increased the production of several cytokines (IFN-γ, TGF-β, TNF-α and IL-12). On the contrary, the cytokine producion of cells from oral squamous cell carcinoma, had largely decreased after RT. These observations suggest that radiation might modulate cytokine production in situ, and therefore, increasing the anti-tumor immune response (46).
Chemokines promote cell trafficking, particularly of leukocytes, but they also participate in local cellular activation and survival. Chemokines can also direct migration of non-immune cells, and can play a major role in invasion by cancer cells (23,24,48). Radiotherapy modulates chemokines, which in turn regulate the tumor microenvironment and its relation with the host immune system (48). IP-10 and MIG are known to exert potent antiangiogenic activities (14). These chemokines recruit T cells to sites of inflammation, providing protective anti-tumor responses. In fact, in humans, it has been shown that tumors with low T cells infiltration are associated with higher expression of angiogenic factors (14,31). Local inflammation induced by radiation enhances the permeability of local vasculature and it also increases the expression of the intercellular adhesion molecule 1 (ICAM-1), E-selectin and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells, facilitating tumor infiltration by immune cells (10,22,31,48,49).
Non-targeted effects: systemic (abscopal effects)
The term “abscopal” derives from the Latin prefix, (“Ab”: means, ‘away from’ and “scopus”: means ‘target’) was first proposed by Mole in 1953. This phenomenon can be described as a tumor response that occurs in non-irradiated cells at a distance from the irradiated site (50) [for a summary of the main characteristics of non-targeted effects (bystander and abscopal effects) induced by RT, see Table 1]. Multiple mechanisms have been proposed to cause the abscopal effects (10,15), such as the systemic secretion of specific cytokines and chemokines, a systemic immune response against local tumor antigens released, or local inflammation that can lead to a distant effect (35). In any case, the hypothesis that the abscopal effect is immune-mediated is becoming stronger. If the dose of radiation is sufficient to generate cell death, this can lead to the induction of the adaptive immune response (Figure 2). RT itself directly elicits an innate immune recognition of tumor, by releasing, ‘‘danger signals’’. Thus, these signals are capable of increasing an immune-mediated cell death that promotes the uptake of circulating tumor antigens by DCs via cross-priming, and ultimately leads to activating tumor-specific T cell response. The tumor-specific T-cells are capable of recirculating throughout the body, detecting any tumor cells eradicating, therefore, tumors that are even at a distant from the irradiated field, which, in turn, is described as an abscopal effect (15,20,34). Some groups have investigated the abscopal effect in several studies using experimental animal models. Preclinical data have demonstrated that local RT can induce a systemic anti-tumor immunity (9,20,21). In a mouse model, Dewan et al. (1) observed that only fractionated, and not RT administered as a single high dose, induced an abscopal effect in a secondary tumor when combined with anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody immunotherapy. Demaria et al. (15) suggested that at least part of the death of tumor cells induced by radiation might be associated with the release of cytokines and other inflammatory stimuli, which can promote the appropriate signals for DCs activation. In a mouse model of mammary carcinoma, these authors demonstrated that T cells are required for the distant effects obtained with the combination of local radiation and Flt-3 Ligand (Flt-3L) treatment (5,15). This would explain why radiation, administered as a single treatment, only results in a few clinically significant abscopal effects (51). Camphausen et al. (35), implicated p53 as a key mediator of the radiation-induced abscopal effect and suggested that it could not be tumor-specific. They found that, high-dose fractions of radiation administered in the normal leg of immunocompetent mice resulted in a reduction of a syngeneic tumor (lung carcinoma or fibrosarcoma) implanted in the dorsal midline. On the contrary, they did not observe an abscopal anti-tumor response in p53 knockout mice or in mice with p53 pharmacologically inhibited. The data provided so far, indicates that radiation can elicit complex responses on tissues, and these responses may have systemic effects, which also depend of immune stimulation and the tumor microenvironment composition (25). However, irradiation of primary tumors may enhance or suppress primary tumor growth and secondary malignancies (52). It has been reported an epidemiological study that evaluated the risk of secondary cancer in patients with a history of prostate cancer radiation. The authors observed an increase in rates of secondary tumors in distant sites, such as the lung. These findings suggest that the abscopal effect induced by radiation could be involved in the clinical outcome of patients treated with local RT. Besides this, there are also documented cases of abscopal effects in normal tissues. Therefore, it is becoming clear that the abscopal effect may be both beneficial for controlling tumor growth and for damaging tissue toxicity (34). The contribution of RT into inducing abscopal anti-tumor immune effects will depend on the ability to alter the pre-existing conditions (immunosuppressive and tolerogenic) in the tumor microenvironment by promoting the proimmunogenic state rather than the immunosuppressive effects (51).
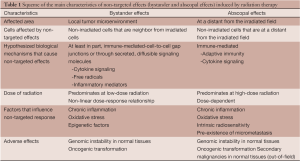
Full table
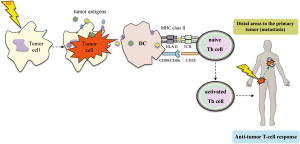
RT and cell death pathways
RT can induce cell death, but it can also enhance the permeability of solid tumors directly or by means of cytokine production that recruit both DCs and effector T cells into the local milieu. Radiation-induced DNA damage can occur through multiple mechanisms (11). Cellular communication elicits a wide variety of responses that have dual biological effects. It can be deleterious (e.g., gene mutation and chromatid exchanges events) or it can be protective through the induction of apoptotic cell death (22). Many factors can influence the fate of cell death pathways that occur after radiation, including type of radiation, radiation dose, tumor type, tumor microenvironment and the host’s immunological characteristics (5). High doses of RT may induce necrotic cell death. RT at local level damages cancer cells, releasing large amounts of tumor antigens in necrotic and apoptotic cancer cells, either alone or in combination with cellular debris, providing signals to effectively activate DCs (Figure 2) (17). The release of tumor antigens upon cancer cell death may help to reestablish tumor-antigen presentation. The radiation-induced tumor cell death enables the presentation of tumor-derived antigens by DCs that may help to elicit a T-cell immune response against the tumor (6,48).
Radiation induce necrotic cell death, especially after delivering high doses (21). Necrotic cell death is considered an immunogenic pathway if accompanied by the release of several stress signals (26). Necrosis comes together with the release of pro-inflammatory cytokines (IL-8, TNF-α) and damage-associated molecular patterns (DAMPs), such as the high-mobility group box 1 protein (HMGB1). The release of HMGB1 from necrotic and apoptotic cells stimulates the TLR4 receptor on DCs, and hence, promotes tumor cell killing by the induction of anti-tumor T cell response. Apetoh et al. (53). demonstrated that HMGB1 released by dying tumor cells, triggered a protective anti-tumor immunity throughout the TLR4-myeloid differentiation primary response protein-88 (MyD88) signaling pathways that are required for the efficacy of CT and RT in mice. The authors found that Asp299Gly TLR4 mutation has a dominant-negative effect on the TLR4/HMGB1 interaction in human patients with breast cancer and compromises the efficacy of antitumor CT. These findings suggest that TLR4 signaling may affect clinical outcome in patients (53,54). After irradiation, tumor cells can also release other “danger” signals such as heat shock proteins (HSPs) (6). When Schildkopf et al. (55) combined RT and additional stress stimuli such as hyperthermia (41.5 °C for 1 h) in colorectal cells, they detected a cell cycle arrest of the necrotic tumor cells in the G2-phase. They also observed a release of cellular components including certain DAMPs (HMGB1, HSP70) that lead to DC activation (55,56).
Radiation also induces apoptosis, which usually occurs at lower doses of irradiation (3,9,10,21). Although apoptosis is considered a non-inflammatory process, radiation-damaged cells may increase the release of inflammatory cytokines and DNA-damaging free radicals (43). It has also been demonstrated that antigens released by apoptotic cell death induced by IR may be immunogenic (2). Apoptotic cells, in the presence of inflammatory signals, induce the production of IL-6 and TGF-β, and promote the development of Th17 cells. By contrast, TGF-β, in absence of inflammation, and particularly of IL-6, induces differentiation of Treg cells. After irradiation, in an anti-inflammatory milieu, with low or absence of IL-6 levels, TGF-β would promote the maintenance of Treg cell differentiation, which inhibits the anti-tumor response (21).
Cancer cells also die by autophagy, another cell death mechanism of tumor cells. It is a catabolic lysosomal mechanism involved in self-digestion of dysfunctional cellular components (57,58). In tumor cells, autophagy induced by IR can release adenosine triphosphate (ATP), critical for the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation in macrophages, that cause IL-1β production (59) and CD8+ T cell polarization. As is widely known, IL-1β regulates many cellular processes, and its secretion from stimulated DCs is largely dependent on the NLRP3 inflammasome (21,43,59).
Although necrosis was regarded as an unregulated and uncontrolled form of cell death, recent evidence shows that it can also occur as a programmed cell death which has been termed as necroptosis (60,61). Necroptosis leads to rapid plasma membrane rupture, swelling of organelles, the release of intracellular contents and exposure of DAMPs (62). Among the molecules involved in the initiation of necroptosis is the receptor interacting protein 1 (RIP1). RIP1 is required for necrosome formation and, therefore, is critical for the activation of necroptosis (60). As mentioned above, a combination of radiation-induced DNA-damage and hyperthermia provokes an immunogenic cell death mechanism such as necrosis, but also by means of necroptosis (55).
Mitotic catastrophe is considered the major mode of death in response to DNA-damage induced by radiation in cells that have impaired the machinery to repair DNA (e.g., cells with defective in p53). It occurs during or as a result of aberrant mitosis due to improper entry into mitosis and leads to the formation of giant cells with aberrant nuclear morphology, centrosome hyperamplification, multiple nuclei, and micronuclei (61,63). These cells may survive through several cycles of cell division, transit into senescence, or die by delayed apoptosis or delayed necroptosis/necrosis (63).
High dose radiotherapy
As previously mentioned, the effects of radiation will depend on several factors. Radiation doses and tissue type influence the local response and, together with other factors such as the genetic background of the host and the inborn characteristics of tumor cells, it could modulate the systemic response into a pro- or anti-tumor effect (5,29).
Data based on preclinical studies have suggested that RT, especially with higher single doses, can stimulate anti-tumor T cell immunity by promoting the cross-priming of antigen-specific DCs increasing the number of activated CD8+ T cells (1,4,10). Particularly, some systemic effects have been related to the high-dose stereotactic ablative body radiotherapy (SABR), which could be used to enhance the production of tumor antigen-specific cellular immunity (10). Thus, a single high dose of 20-25 Gy, can substantially increase the T-cell response and help to control tumor growth (6). In humans, Postow et al. (64) observed a case of abscopal effect in a patient with metastatic melanoma. The patient was treated with ipilimumab [anti-CTLA-4 antibody] and the tumor regression was seen only after the combination with RT using a dose-fractionation schedule. CTLA-4 is expressed on activated T cells, which provide inhibitory signals to T cells. Blocking of CTLA-4 promotes T cell activation. The authors found a temporal association between tumor shrinkage and antibody response to cancer-testis antigen NY-ESO-1. Also, they analyzed the immune cells of peripheral blood. Before RT administration, they observed an increase of activated CD4+ T cell population during the treatment with ipilimumab, and a second increase was also observed after RT. In addition, an enhancement of HLA-DR expression was observed on CD14+ monocytes after RT. On the contrary, the levels of suppressor MDSCs (CD14+ HLA-DRlow) decreased. It has been shown that MDSCs are expanded in patients with metastatic melanoma, whilst not effectively detected in healthy controls (65). These results lead the authors to conclude that RT has an immunomodulatory role which would act by promoting the expansion of activated T-cells, increasing the presentation of antigens by myeloid cells within the tumor stroma, thus promoting T-cell function to kill tumor cells. Consequently, it can be argued that the abscopal effect is mediated by adaptive immunity (64). At high doses, the involvement of the microenvironment in radiation effects could be due in part to changes generated in the local irradiated tissue, preventing tumor recurrence or metastasis. But, at low doses, under conditions of chronic exposure to radiation, there is a complex interplay of diverse modulating factors and the microenvironment could provide compensation to direct damages on DNA induced by radiation (43). In a recent study, Hei et al. (66) debated the possible association between the non-targeted response and secondary cancer induced by radiation. Cells stressed by low-dose irradiation create a chronic inflammatory milieu with specific cytokines and reactive radical species which can generate secondary genotoxic effects that may affect both the surrounding non-irradiated cells and distant normal tissues (5,34).
TARGIT and immune system
It has been shown that circulating tumor cells are able to reinstate on the primary tumor site and promote its growth. This may present a risk for local recurrence within the primary site (and distant metastasis) (43,67). Advances in RT techniques allow the use of high-dose RT (10-20 Gy) which applied on tumor bed, may reduce the risk of a local relapse (67,68). Some reports suggest that high-dose RT can induce unexpected indirect effects both at local level (bystander effect) and outside the irradiated field (abscopal effect) (19,23).
In breast cancer, when radiotherapy is administered in combination with surgery, the risk of local recurrence is dramatically reduced within the primary site (43). Targeted intraoperative radiotherapy (TARGIT) may reduce tumor recurrence modifying the wound microenvironment when is applied immediately following excision (19,43,69). This technique delivers therapeutic radiation to the tissues around the primary tumor during breast-conserving surgery (BCS), adding 20-40 min to the operation time (70-72). TARGIT using a mobile device called INTRABEAM® (a miniature X-ray source with 50 kV) delivers 20 Gy as one high dose of radiation on tumor bed and decreases faster to 5-7 Gy at 1 cm into the surrounding tissue. Thus, the volume of breast tissue that receives a high dose of radiation is much lower and allows normal tissues are repaired during intraoperative radiotherapy (IORT) (68,72).
In a recent study, Belletti et al. (69) demonstrated that TARGIT modified significantly the protein expression of the wound fluid (WF). In that sense, it is known that WF stimulates proliferation, chemotaxis and invasion of breast cancer cell lines. However, WF from TARGIT-treated patients dramatically reduced the stimulatory effect on cancer cells in vitro. In a proteomic analysis, the authors examined several factors that may be responsible for these effects, particularly whether these factors modified by TARGIT may be involved in the control of cancer cell progression. The WF from TARGIT-treated patients showed a modified expression of certain cytokines, and lost the ability to induce the activation of some intracellular signal transduction pathways. They observed that several factors (e.g., IL-6, RANTES or leptin) and pathways (e.g., STAT3- and p70S6 kinase-mediated pathways), involved in controlling tumor cell growth and motility, decreased after TARGIT treatment (69). IL-6 activates STAT-3, a member of the STAT family of transcription factors, which control a wide variety of cellular processes and are involved in signaling by many cytokine receptors. In in vitro experiments, it has been shown that STAT-3 activation promotes cell migration and metastasis of breast cancer cells (73). Lower levels of several growth factors and chemokines can inhibit angiogenesis. In the WF, TARGIT induced a decreased expression of several chemokines (e.g., MCP-1 and IL-8), as well as in vascular endothelial growth factor (VEGF), but caused an increase of granulocyte colony-stimulating factor (G-CSF) (69). It was hypothesized that tumor-derived G-CSF promotes the development of MDSCs, a heterogeneous population of immature myeloid cells that accumulates in the tumor-bearing host and has the ability to suppress T cell responses (74). In mouse models, it has been shown that inappropriate production of G-CSF contributes to MDSCs accumulation (75). In the tumor microenvironment, MDSCs can be immunosuppressive, e.g., suppressing effector T cells and NK cell functions. Some data suggest that these suppressor cells may promote the expansion of Treg cells and hence, suppressing the responses of other immune cells (76). Moreover, the authors observed an increase of Th cell-derived cytokines, which is reminiscent of a Th2 profile (IL-13, IL-4, IL-5). Th2-derived cytokines have been shown to promote the differentiation of “tumor-promoting M2 macrophages” also called “alternative macrophages” that express an anti-inflammatory cytokines, such as TGF-β and IL-10 (77). These macrophages are associated with tumor progression (21,46), as a result of pro-angiogenic factors and their immune-suppressive function (27,49). These findings indicate that WF generated after surgery procedures may act through immunological mechanisms, increasing the levels of growth factors while decreasing the activation of the immune system. The enhanced production of these mediators could suppress the immune response, altering cells and the local microenvironment and promoting the recruitment of residual tumor cells. However, WF from TARGIT-treated patients dramatically reduced the stimulatory effect on cancer cells reducing tumor recurrence. These preliminary results pave the way for future studies aimed to know the role of the local microenvironment in tumor development.
Concluding remarks and perspectives
Although cancer progression is mainly driven by the expansion of tumor cells, tumor microenvironment and anti-tumor immunity are recognized as important factors for control of tumor growth. Exciting and promising results implying RT as an inductor of tumor immunogenicity, even at distant metastatic sites, have been reported over the last decade. As a result, RT could render cancer cells visible to the immune system.
RT not only leads to DNA damage in tumor cells, but it also might alter tumor microenvironment. Consequently, the low recurrences observed with TARGIT could be, at least in part, related to the effects of RT on the molecular composition and biological activity of WF and its effects on tumor cells and in the immune system. However, the nature of the mediators presented in the WF remains largely unknown and no studies have investigated the effects of TARGIT on cellular immunity.
Soluble mediators, particularly cytokines and chemokines released by tumor and non-tumor cells in response to RT, may affect tumor growth. However the pro- or anti-tumor effects of many cytokines or chemokines is largely unknown. Inflammation triggers and amplifies innate immunity and also collaborates to the development of adaptive, specific, immunity. On the other hand, inflammation may also exert a pro-tumor effect. In fact, even the same mediator may exert pro or anti-tumor effects and hence, in a clinical setting, it could be a double-edged sword. Certainly, more information is needed before drawing firm conclusions about the role of several cytokines in tumor development.
It would be interesting to understand how the tumor milieu originated by RT contributes to cancer progression. This knowledge would pave the way for the development of innovative strategies aimed at achieving a more effective anti-tumor microenvironment; particularly how mediators induced by RT modify tumor growth and anti-tumor responses.
In a clinical context, the goal of RT is to cause the maximum permanent damage on patient’s tumors while minimizing the risk of harm to a patient’s normal tissues. There is evidence of cases of abscopal effects reported in normal tissues. Since these effects induced by radiation may enhance or suppress the growth of primary tumors and secondary malignancies, epidemiological approaches are necessary to evaluate the benefit and damage associated with these biological effects.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frederik Wenz and Elena Sperk) for the series “Intraoperative Radiotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.02.05). The series “Intraoperative Radiotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [PubMed]
- Lara PC, López-Peñalver JJ, Araujo Farías V, et al. Direct and bystander radiation effects: a biophysical model and clinical perspectives. Cancer Lett 2013. pii: S0304-3835(13)00659-9.
- Levy A, Chargari C, Cheminant M, et al. Radiation therapy and immunotherapy: implications for a combined cancer treatment. Crit Rev Oncol Hematol 2013;85:278-87. [PubMed]
- Frey B, Rubner Y, Kulzer L, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother 2014;63:29-36. [PubMed]
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718-26. [PubMed]
- Zeng J, Harris TJ, Lim M, et al. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int 2013;2013:658126.
- Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol 2005;83:451-61. [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [PubMed]
- Kaminski JM, Shinohara E, Summers JB, et al. The controversial abscopal effect. Cancer Treat Rev 2005;31:159-72. [PubMed]
- Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol 2012;2:191. [PubMed]
- Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002;62:1462-70. [PubMed]
- Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004;64:4328-37. [PubMed]
- Desai S, Kumar A, Laskar S, et al. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine 2013;61:54-62. [PubMed]
- Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol 2001;166:2276-82. [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [PubMed]
- Morgan WF, Sowa MB. Non-targeted effects induced by ionizing radiation: Mechanisms and potential impact on radiation induced health effects. Cancer Lett 2013. pii: S0304-3835(13)00662-9.
- Lumniczky K, Sáfrány G. Cancer gene therapy: combination with radiation therapy and the role of bystander cell killing in the anti-tumor effect. Pathol Oncol Res 2006;12:118-24. [PubMed]
- Campa A, Balduzzi M, Dini V, et al. The complex interactions between radiation induced non-targeted effects and cancer. Cancer Lett 2013. pii: S0304-3835(13)00699-X.
- Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 2009;9:351-60. [PubMed]
- Frey B, Rubner Y, Wunderlich R, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Curr Med Chem 2012;19:1751-64. [PubMed]
- Lumniczky K, Sáfrány G. The impact of radiation therapy on the antitumor immunity: Local effects and systemic consequences. Cancer Lett 2013. pii: S0304-3835(13)00599-5.
- Kadhim M, Salomaa S, Wright E, et al. Non-targeted effects of ionising radiation-Implications for low dose risk. Mutat Res 2013;752:84-98. [PubMed]
- Mothersill C, Seymour CB. Radiation-induced bystander effects--implications for cancer. Nat Rev Cancer 2004;4:158-64. [PubMed]
- Butterworth KT, McMahon SJ, Hounsell AR, et al. Bystander signalling: exploring clinical relevance through new approaches and new models. Clin Oncol (R Coll Radiol) 2013;25:586-92. [PubMed]
- Rödel F, Frey B, Multhoff G, et al. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett 2013. pii: S0304-3835(13)00669-1.
- Tesniere A, Panaretakis T, Kepp O, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008;15:3-12. [PubMed]
- Sprung CN, Ivashkevich A, Forrester HB, et al. Oxidative DNA damage caused by inflammation may link to stress-induced non-targeted effects. Cancer Lett 2013. pii: S0304-3835(13)00661-7.
- Fernandez-Palomo C, Schültke E, Smith R, et al. Bystander effects in tumor-free and tumor-bearing rat brains following irradiation by synchrotron X-rays. Int J Radiat Biol 2013;89:445-53. [PubMed]
- Munro AJ. Bystander effects and their implications for clinical radiotherapy. J Radiol Prot 2009;29:A133-42. [PubMed]
- Disis ML, Stanton SE. Can immunity to breast cancer eliminate residual micrometastases? Clin Cancer Res 2013;19:6398-403. [PubMed]
- Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 2008;180:3132-9. [PubMed]
- Eljaszewicz A, Wiese M, Helmin-Basa A, et al. Collaborating with the enemy: function of macrophages in the development of neoplastic disease. Mediators Inflamm 2013;2013:831387.
- Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res 2012;178:505-23. [PubMed]
- Siva S, Macmanus MP, Martin RF, et al. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2013. pii: S0304-3835(13)00672-1.
- Camphausen K, Moses MA, Ménard C, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res 2003;63:1990-3. [PubMed]
- Cárdenes M, Angel-Moreno A, Fieschi C, et al. Oesophageal squamous cell carcinoma in a young adult with IL-12R beta 1 deficiency. J Med Genet 2010;47:635-7. [PubMed]
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon gamma receptor 2 (IFN gamma R2) deficiency. J Med Genet 2010;47:631-4. [PubMed]
- Bax HI, Freeman AF, Anderson VL, et al. B-cell lymphoma in a patient with complete interferon gamma receptor 1 deficiency. J Clin Immunol 2013;33:1062-6. [PubMed]
- Bonavina L, Incarbone R, Reitano M, et al. Candida colonization in patients with esophageal disease: a prospective clinical study. Dis Esophagus 2003;16:70-2. [PubMed]
- Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 1998;95:7556-61. [PubMed]
- Peiser M. Role of Th17 cells in skin inflammation of allergic contact dermatitis. Clin Dev Immunol 2013;2013:261037.
- Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature 2006;442:461-5. [PubMed]
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer 2005;5:867-75. [PubMed]
- Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity 2013;38:414-23. [PubMed]
- Torchinsky MB, Garaude J, Martin AP, et al. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 2009;458:78-82. [PubMed]
- Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine 2008;43:374-9. [PubMed]
- Yamamoto T, Kimura T, Ueta E, et al. Characteristic cytokine generation patterns in cancer cells and infiltrating lymphocytes in oral squamous cell carcinomas and the influence of chemoradiation combined with immunotherapy on these patterns. Oncology 2003;64:407-15. [PubMed]
- Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res 2008;10:215. [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [PubMed]
- Mole RH. Whole body irradiation-radiobiology or medicine? Br J Radiol 1953;26:234-41. [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [PubMed]
- Bostrom PJ, Soloway MS. Secondary cancer after radiotherapy for prostate cancer: should we be more aware of the risk? Eur Urol 2007;52:973-82. [PubMed]
- Apetoh L, Tesniere A, Ghiringhelli F, et al. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008;68:4026-30. [PubMed]
- Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13:1050-9. [PubMed]
- Schildkopf P, Frey B, Mantel F, et al. Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem Biophys Res Commun 2010;391:1014-20. [PubMed]
- Schildkopf P, Frey B, Ott OJ, et al. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol 2011;101:109-15. [PubMed]
- Ma Y, Galluzzi L, Zitvogel L, et al. Autophagy and cellular immune responses. Immunity 2013;39:211-27. [PubMed]
- Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer 2013;12:171. [PubMed]
- Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009;15:1170-8. [PubMed]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, et al. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010;11:700-14. [PubMed]
- Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009;16:3-11. [PubMed]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 2013;38:209-23. [PubMed]
- Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol 2010;31:363-72. [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [PubMed]
- Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007;25:2546-53. [PubMed]
- Hei TK, Zhou H, Chai Y, et al. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol 2011;4:96-105. [PubMed]
- Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. [PubMed]
- Tuschy B, Berlit S, Romero S, et al. Clinical aspects of intraoperative radiotherapy in early breast cancer: short-term complications after IORT in women treated with low energy x-rays. Radiat Oncol 2013;8:95. [PubMed]
- Belletti B, Vaidya JS, D’Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008;14:1325-32. [PubMed]
- Wenz F, Blank E, Welzel G, et al. Intraoperative radiotherapy during breast-conserving surgery using a miniature x-ray generator (Intrabeam®): theoretical and experimental background and clinical experience. Womens Health (Lond Engl) 2012;8:39-47. [PubMed]
- Vaidya JS, Baum M, Tobias JS, et al. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol 2001;12:1075-80. [PubMed]
- Vaidya JS, Baum M, Tobias JS, et al. Long-term results of targeted intraoperative radiotherapy (Targit) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2011;81:1091-7. [PubMed]
- Snyder M, Huang XY, Zhang JJ. Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration. J Biol Chem 2011;286:38886-93. [PubMed]
- Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol 2011;13:591-9. [PubMed]
- Waight JD, Hu Q, Miller A, et al. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One 2011;6:e27690 [PubMed]
- Nagaraj S, Gabrilovich DI. Regulation of suppressive function of myeloid-derived suppressor cells by CD4+ T cells. Semin Cancer Biol 2012;22:282-8. [PubMed]
- Hao NB, Lü MH, Fan YH, et al. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012;2012:948098.


