
Harnessing cerium oxide nanoparticles to protect normal tissue from radiation damage
Introduction
Free radicals are formed through ionizing reactions, such as the photoelectric, Compton and Auger effects. These free radicals react with DNA and RNA, causing molecular alterations, improper segregation of chromosomes during mitosis, and radiation-induced mitotic death (mitotic catastrophe) (1,2). Furthermore, radiation-induced cellular oxidative damage is initiated by the generation of reactive oxygen species (ROS), which are known to change the oxidative status of cells, resulting in changes in mitochondrial function and activation/inactivation of various proteins involved in the apoptosis (cell death) process (3). When healthy (normal) cells are exposed to radiation, they ameliorate the damaging effect of free radicals by the release of innate protective molecules such as superoxide dismutase (SOD), glutathione, and metallothionine, which increase and intensify DNA repair mechanisms (3). Nonetheless, while these protective and repair mechanisms for cells are efficient, they are not capable of blocking all of the damage, which ultimately leads to normal tissue death.
In an effort to combat the harmful effects of radiation, various free radical scavengers have been tested for their ability to protect normal cells and tissues. Free radical scavengers such as Amifostine, Vitamin E, ascorbate, carotenes, melatonin and lipoic acid derivatives are the subject of many recent reviews (4). However, many of these free radical scavengers were found to have limited success due to short half-lives (hours or even minutes), lack of penetration to the site of radical production, and daily dosing requirements. This report discusses a novel approach for the protection of normal cells against radiation-induced cell damage by using cerium oxide (CeO2) nanoparticles.
Most recently, CeO2 nanoparticles have been tested for their ability to serve as free radical scavengers (5-7) to render protection against chemical, biological and radiological insults that promote the production of free radicals. The chemistry of engineered CeO2 nanoparticles supports a potential role as a biological free radical scavenger or antioxidant. It was suggested that the unique structure of CeO2 nanoparticles, with respect to valence and oxygen defects, promotes cell longevity and decreases toxic insults by virtue of its antioxidant properties that occurs when the nanoparticles enter the cells (8), prevent the accumulation of ROS and thereby preventing the activation of the apoptotic response and death of the cells (5).
In this report, CeO2 nanoparticles are shown to confer protection against radiation-induced cell damage in vitro and in vivo, suggesting that CeO2 nanoparticles are an effective radioprotectant for normal tissues.
Radiotherapy side effects
No cancer treatment is without side effects. Following radiotherapy, many patients experience side effects such as mild neutropenia, swelling or pain, and telangiectasia (a sunburn-type appearance of the skin); however these early side effects usually disappear within several weeks. Early side effects occur in rapidly proliferating tissues, and are generally not dose-limiting factors, and have minimal long term impact upon the quality of life (QOL) of the patient. Of far greater concern, is the emergence of late-reacting tissue damage in organs such as the lungs, skin and spinal cord; radiation damage to such tissues manifests itself weeks to months after the completion of therapy. These severe normal tissue reactions cause extensive discomfort to the affected individuals and limit the radiation dose that can be delivered to the entire patient population.
CeO2 nanoparticles as radioprotectants
Nanotechnology is a multidisciplinary field that involves the design and engineering of objects <100 nanometers (nm) in size. A new generation of free radical scavengers is nanoparticles. The role of nanoparticles as radioprotectants is a cutting-edge development addressing decades of scientific interest regarding the protection of normal cells and tissues from radiation. The chemistry of engineered CeO2 nanoparticles supports a potential role as a biological free radical scavenger or antioxidant. Current studies highlighted in this chapter suggest that nanoparticles may be a therapeutic regenerative material that will scavenge ROS that are responsible for radiation-induced cell damage.
CeO2 nanoparticles in biological applications
While there are some concerns about the toxicity of nanoparticles, there are very few reports regarding the biologically detrimental effects of CeO2 nanoparticles. In an article published recently in Toxicology, Park et al. conclude that CeO2 nanoparticles (15-45 nm; 5-40 µg/mL) induced oxidative stress and cell death in cultured human lung epithelial cells (9). It is important to note that these particles are significantly larger than the nanoparticles used in the experiments discussed because the size of a nanoparticle affects the free radical scavenging ability of the particle by modifying the ratio of cerium (III) to cerium (IV). Furthermore, Park et al. exposed the cells to CeO2 nanoparticles doses ~1,000 times the effective radioprotective dose was recently published (6).
Need for better radioprotective compound
Free radical scavengers such as Amifostine, Vitamin E, ascorbate, carotenes, melatonin and lipoic acid derivatives possess few active sites per molecule. A more recently investigated antioxidant, C60, may be able to scavenge a comparatively more number of radicals than the currently available antioxidants (10). But, due to the limited number of free radical scavenging sites, repeated dosing is required to replace molecular species that were utilized in free radical reduction. However, CeO2 nanoparticles offer many active sites for free radical scavenging due to their large surface to volume ratio and, more importantly, due to their mixed valence states for unique redox chemistry. A recent article reports SOD mimetic activity of CeO2 (11). Additionally, the free radical scavenging property of CeO2 nanoparticles is regenerative (6) which is not the case for other antioxidants. It is believed that due to the chemical nature of CeO2 nanoparticles, there is an auto-regenerative reaction cycle (Ce3+→Ce4+→Ce3+) continuing on the surface of ceria nanoparticles and is thought to be the current mechanism by which it provides the material with an unprecedented free radical scavenging ability (Figure 1).
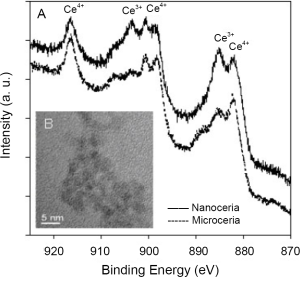
CeO2 nanoparticles exhibit in vitro free radical scavenging ability
The chemistry of engineered CeO2 nanoparticles supports their potential role as free radical scavengers, antioxidants, in biological systems (10). It was suggested that the unique surface chemistry of CeO2 nanoparticles, with respect to valence and oxygen defects, decreases oxidative insults by virtue of its antioxidant properties and promotes cell longevity. Thus far, studies have shown that CeO2 nanoparticles enter mammalian cells (8), decrease the accumulation of ROS, and prevent the activation of the ROS-induced apoptosis (5). Since cells produce ROS after being exposed to radiation (12), the antioxidant capability of CeO2 nanoparticles has been suggested as the key mechanism by which CeO2 nanoparticles confers radioprotection (6). Furthermore, a study concluded that CeO2 nanoparticles exhibited SOD-mimetic activity (12). Results supporting the antioxidant properties of CeO2 nanoparticles is mounting, and many studies suggest that these nanoparticles act as free radical scavengers (6,7,13) and may render protection against chemical insults that promote the production of free radicals (14). Thus, it has been proposed that CeO2 nanoparticles may confer radioprotection by scavenging the free radical produced during radiotherapy (6).
CeO2 nanoparticles protect mice from total body irradiation (TBI)
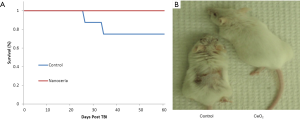
Balb-C mice were randomized into 2 groups (n=10). Group 1 was injected with saline (control group). Group 2 received a total CeO2 nanoparticles dose of 0.005 mg/kg. On day 5, all animals received 12.5 Gy of X-ray radiation. No animals died in the CeO2 nanoparticles group during the first 60 days post irradiation. In sharp contrast, 20% of the control animals died (Figure 2A). During the experiment we observed that many of the control animals appeared exhibited skin desquamation, while the CeO2 nanoparticles-treated animals had little skin damage (Figure 2B). These results suggest that CeO2 nanoparticles are able to protect mice from a single dose of radiation, and support CeO2 nanoparticles’s role as a radioprotectant (15).
CeO2 nanoparticles is well-tolerated in athymic mice
To investigate the acute toxicity of CeO2 nanoparticles, athymic nude mice were randomized into five groups. Each group received a total nanoparticle dose in the range of 0 (saline), 0.135, 1.35, 13.5, or 135 mg/kg. The mice were observed over a three-week period. No mice died or experienced notable side effects during the treatment. At the end of the treatment, the mice were sacrificed. During necropsy no abnormal pathologies were observed. This indicates that CeO2 nanoparticles are well-tolerated in mice up to 3 million times the effective dose. Therefore, it was suggested that CeO2 nanoparticles causes limited toxicity and side effects in mice (15).
Applications to areas of health and disease
When biological systems are under high energy exposure ROS are produced at high levels and cellular components can be damaged. These ROS can be used by biological systems as a defense mechanism against microorganisms and can act as signal transduction and transcription agents in development, stress responses, and programmed cell death. Oxidative stress arises from the strong cellular oxidizing potential of excess ROS, or free radicals. In addition, elevated levels of oxidative damage are related to increased risks for cataracts, cardiovascular disease, and cancer.
Therefore, the potential benefit of radioprotection using CeO2 nanoparticles is of great significance on multiple levels—the most important is its potential impact on human life. This research is relevant to the health and QOL of humans worldwide who are exposed to radiation environments such as those listed below:
- Patients receiving radiation treatments for cancer;
- Astronauts in NASA exposed to particle radiation;
- Military and civilians potentially exposed to radiation in battle, terrorism or occupational exposure.
Verification of the effectiveness of nanoparticles as radioprotectors opens the field for future studies that would examine, in depth, the mechanism, tissue distribution and safety of CeO2 nanoparticles, prior to utilization in Phase I clinical trials. In the end, these studies may lead to faster recovery and improved QOL for the patients suffering from radiation damage.
Protection of radiation-induced pneumonitis using CeO2 nanoparticles
Radiotherapy as a treatment for lung cancer
Radiotherapy is an effective treatment option for lung cancer. However, lung tissue is particularly sensitive to radiation. Thus, the efficacy of radiotherapy is limited by the low tolerance of lung tissue to radiation exposure, and medical professionals seek to optimize the ratio of tumor debulking to lung toxicity. Unfortunately, 30% of patients that receive radiation during their treatment for lung cancer experience clinically significant lung injury (16), and there is no effective therapeutic available for the prevention of acute or chronic radiation-induced pneumonopathy (17). The availability of a radioprotective therapeutic that selectively protects normal lung tissue from radiation-induced-damage would significantly improve the ability of medical professionals to treat patients with lung cancer.
CeO2 nanoparticles exhibit selective radioprotection of lung fibroblasts in vitro
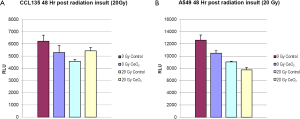
Normal lung fibroblasts (CCL-135), pre-treated with CeO2 nanoparticles (10 nM) were exposed to 20 Gy. A Cell Titer-Glo Luminescent Cell Viability Assay (which signals the presence of metabolically active cells) was performed 48 hours after irradiation, and the irradiated normal lung fibroblasts that received CeO2 nanoparticles pre-treatment had increased viability when compared to irradiated normal cells that did not receive CeO2 nanoparticles treatment (Figure 3A). When the same experiment was performed on a non-small cell lung cancer cell line (A549), there was no protection (Figure 3B) (15).
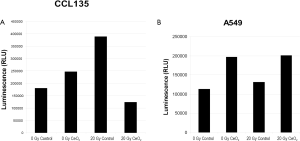
In a similar study, normal lung fibroblast (CCL 135) and lung cancer cells (A549) were pretreated with 10 nM CeO2 nanoparticles for 24 hours. Cells were then irradiated with 20 Gy and incubated for 48 hours and assayed for Caspase3/7 activity, which is a protein that is activated during apoptosis. In the presence of CeO2 nanoparticles, normal cells did not undergo radiation-induced apoptosis (Figure 4A). In sharp contrast, CeO2 nanoparticles did not protect the A549 cells from radiation-induced apoptosis (Figure 4B) (15).
Radiation-induced damage and oxidative stress are closely tied. Irradiated cells produce damaging ROS. Previous studies show that CeO2 nanoparticles exhibits SOD-mimetic activity. To investigate whether CeO2 nanoparticles can decrease intracellular ROS post irradiation, normal lung fibroblasts were treated with CeO2 nanoparticles (10 nM) for 24 hours and then irradiated (20 Gy). Intracellular ROS was imaged using the Image-iT Live Green Reactive Oxygen Species Detection Kit. Control cells were irradiated in the absence of CeO2 nanoparticles (Figure 5A). Results show that CeO2 nanoparticles decreased the radiation-induced accumulation of ROS (Figure 5B). These in vitro results show that CeO2 nanoparticles selectively conferred protection against radiation-induced cell death in normal cells (and not cancer cells) (15).
CeO2 nanoparticles treatment decreases radiation-induced pneumonitis in murine model
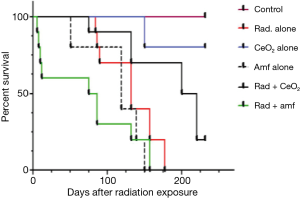
Radiation pneumonitis and subsequent pulmonary fibrosis can significantly decrease the QOL of humans exposed to radiation. In an attempt to administer nanoparticles to live animals and to evaluate the radiation protection activity of CeO2 nanoparticles, the survival of non-tumor bearing athymic nude mice was measured. Non-tumor bearing athymic nude mice were exposed to fractionated doses of 30 Gy radiation (weekly administration of 5 Gy) in the presence or absence of twice weekly i.p. injections of CeO2 nanoparticles or i.p. injections of Amifostine 30 minutes prior to radiation. Results show (Figure 6) that CeO2 nanoparticles are well tolerated by athymic nude mice and protect mice from radiation-associated death. All control mice lived until termination date of 231 days. In mice treated with CeO2 nanoparticles alone, 20% were sacrificed on day 150 for histology analysis. The remaining 80% were alive until the termination date of 231 days. After treatment with radiation alone, Amifostine alone, and a combination of radiation and CeO2 nanoparticles, or radiation and Amifostine, the median survival time was 132, 119, 225, and 81 days, respectively (control versus radiation, P<0.019; control versus CeO2, P<0.66; control versus Amifostine, P<0.0370; radiation versus radiation and CeO2, P<0.0041; radiation versus radiation and Amifostine, P<0.0432). In contrast, Amifostine was highly toxic, as shown by the significant difference in median survival time (as compared to control mice). In summary, these results suggest that CeO2 nanoparticles are well tolerated by mice and have a significant advantage over the clinically used Amifostine (15).
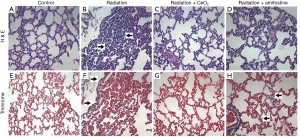
To determine the degree of radiation-induced pneumonitis, the lungs were harvested and processed for histology and hematoxylin and eosin (H&E) staining. The lungs from mice in the control group (radiation alone) showed visible pneumonitis, with extensive macrophage invasion; whereas the lungs from irradiated mice receiving CeO2 nanoparticles showed no visible pneumonitis and appeared normal (Figure 7). In addition, the amount of fibrosis and collagen deposition (indicative of chronic lung conditions) was measured in the lungs of control mice (no radiation/normal lungs), or in lungs of those mice treated with radiation alone, radiation plus CeO2, or radiation plus Amifostine, using Masson’s Trichrome stain. The histology analyses show that fibrosis and collagen deposition were common in the irradiated lungs of those mice given radiation alone and of those mice given a pretreatment of Amifostine (Figure 7). Furthermore, analysis indicated that collagen deposits were relatively recent, due to the faint blue stain, as compared to dark blue staining of older, more cross-linked collagen seen in human chronic lung diseases. In sharp contrast, no significant Trichrome staining was observed in normal lungs (control) or in those irradiated lungs of mice treated with CeO2 (15).
CeO2 nanoparticles treatment reduces over-expression of Transforming Growth Factor-beta (TGF-β), a marker for fibrosis
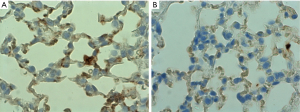
Athymic mice were randomized into two groups. Group 1 received 0.005 mg/kg of CeO2 nanoparticles prior to irradiation, while group 2 received saline. The mice were irradiated in the ventral thorax with 30 Gy X-rays (fractionated into 5 doses over two weeks). The mice were sacrificed 120 days after irradiation, and the lungs extracted for immunohistochemistry. Slides of lung tissue were stained using a primary antibody (monoclonal mouse anti-mouse TGF-β1 and secondary antibody (goat anti-mouse HRP), and the slides were counterstained with hematoxylin. The stained slides were imaged with light microscope using oil immersion at 1,000× (Figure 8). The images demonstrate a significant level of TGF-β expression in lungs of the untreated animals. Since high levels of TGF-β expression is linked to lung fibrosis and pneumonopathy (17), the decrease in TGF-β expression in the animals that received CeO2 nanoparticles treatment (as compared to control) indicates that CeO2 nanoparticles protected the mice from radiation-induced pneumonopathy (15).
Harnessing nanoparticles to improve toxicity after head and neck radiation
Radiation therapy has been a major modality employed in the treatment of head and neck cancer for decades. Unfortunately, the tissues in the head and neck region are exquisitely sensitive to the acute and late effects of radiation treatment (18,19). Due to these toxicities, head and neck cancer patients have a uniquely difficult time during a course of radiation. Many patients will require hospitalization, feeding tube placement, pain medications, and intravenous hydration in order to complete the prescribed course of treatment. Moreover, these patients often face long-term difficulties with eating, speaking, tasting, dry mouth, decreased range of motion, and wound healing (20). The need to improve toxicity associated with the radiotherapeutic treatment of head and neck cancer is significant.
Recently published American Society of Clinical Oncology (ASCO) guidelines state that Amifostine “may be considered during fractionated radiation therapy (21).” However, these guidelines do not support the use of Amifostine in the use of concurrent chemoradiation, which is presently the standard of care in the treatment of many head and neck cancer patients (21). Moreover, the ability of Amifostine to ameliorate radiation induced dermatitis and mucositis has not been adequately established (21). Hence, there remains a substantial clinical need for a radioprotective agent that can be delivered with relative ease, is long lasting, well-tolerated, and can protect a spectrum of sensitive normal tissues that are responsible for a significant reduction in QOL. In the present report, we show that CeO2 nanoparticles represent a novel approach to the protection of salivary and skin tissue from radiation-induced damage and report their efficacy as a new radioprotective compound on athymic nude mice receiving radiotherapy to the head and neck.
Effects of CeO2 nanoparticles on athymic nude mice exposed to radiation to the head and neck region
Sialometry analysis demonstrated a statistically significant difference in salivary flow production between the control group that received 30 Gy/6 fractions of radiation and mice treated with 30 Gy/6 fractions of radiation that received concomitant treatment with CeO2 nanoparticles (Figure 9A). The mean stimulated salivary flow rate for the non-radiated group was 313.691 µL/10 min, while the radiated control group had a mean salivary flow of 115.257 µL/10 min. Furthermore, the radiated groups that received either low concentration of CeO2 nanoparticles (15 nM) or high concentration of CeO2 nanoparticles (15 µM) had an increase in salivary flow production (mean salivary flow volumes of, 166.825 µL/10 min and 203.925 µL/10 min, respectively) when compared to the “no nanoparticle” radiated group 12 weeks after radiation exposure.
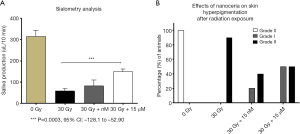
While 100% of the skin hyperpigmentation observed in mice treated with radiation alone was recorded as Grade II, mice treated with 15 nM CeO2 nanoparticles resulted in a lower incidence of grade II (33.33%) and a higher incidence of Grade I (66.67%). In sharp contrast, mice treated with 15 µM CeO2 nanoparticles had an equal incidence of Grade I and II hyperpigmentation (50% each) (Figure 9B). Sialometry analysis demonstrated a statistical significant difference in the stimulated salivary flow, between the radiated control group and the group receiving radiation and 15 µM CeO2 (P value: 0.0003, 95% CI: –128.0 to –52.90).
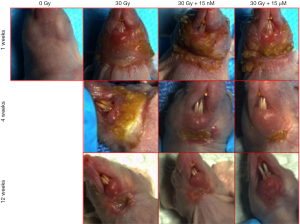
An inverse correlation was observed between the incidence of Grade 3 radiation-induced dermatitis and the concentration of CeO2 nanoparticles given (Figure 10). The incidence of Grade 3 dermatitis 1 week after radiation was decreased in the 15 µM CeO2 group compared to the non-CeO2 controls (10% vs. 100% incidence of Grade 3 dermatitis, respectively). This effect was not appreciated in the 15 nM CeO2 group. Furthermore, animals exposed to radiation and either 15 nM or 15 µM concentration of CeO2 nanoparticles showed swifter resolution of radiation dermatitis when compared to the control “no-nanoparticle” radiated group. For example, complete healing was observed in 60% of animals pre-treated with 15 µM of CeO2 nanoparticles before radiation, vs. 10% on the radiated control group, at 12 weeks post-radiation (Figure 10).
Effects of CeO2 nanoparticles on the apoptotic index of salivary glands parenchymal cells after radiation to the head and neck region
The parotid, sublingual and submandibular glands were independently analyzed and the acinar cell apoptotic index was determined using TUNEL analysis. Our results indicate a dose dependent decrease in the apoptotic index for the individual glands after radiation, indicative of the radioprotective nature of the nanoparticles (Figure 11A). Complementary analysis of the effects of CeO2 nanoparticles combined with radiation on all major salivary gland yielded a similar response (Figure 11B). The overall apoptotic index baseline of acinar cells for the non-radiated group was 1.43%, while radiation-induced damage increased the apoptotic rate to 19.91%. Meanwhile, after treatment with radiation, both (15 nM and 15 µM) CeO2 nanoparticle treated groups exhibited an apoptotic index of 8.17% and 4.67%, respectively. Statistical analysis demonstrated a significant difference between the “no-nanoparticle” treated group and the 15 µM CeO2 treated group (P value: 0.0270, 95% CI: 2.77 to 27.03). Lastly, a comparison between the group that received a combination of nanoparticles plus radiation and the control group (i.e., “no-nanoparticle” “no-radiation” controls) was performed to quantify the degree of radioprotection from apoptotic death compared to virgin salivary tissue. Comparison of the apoptotic index of the 15 µM CeO2 nanoparticle group that received radiation versus the “no-radiation” “no-nanoparticle” control group showed no statistical difference (P value: 0.1155, 95% CI: –8.534 to 1.378).
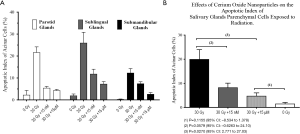
On the other hand, the apoptotic index of the 15 µM CeO2 nanoparticle treated group that did not receive radiation and the non-radiated “no-nanoparticle” control group showed no statistical difference between them. These results suggest that exposure to CeO2 nanoparticles does not result in adverse effects to acinar cells.
CeO2 nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of ROS and upregulation of super oxide dismutase-2
In the context of colorectal carcinomas, damage on surrounding healthy cells which have been inadvertently exposed to ionizing radiation has been exacerbated during radiation treatment since the colon is untethered and mobile, making it particularly susceptible to physical perturbation, such as bladder filling or breathing, which may cause unintended radiation exposure to nearby tissue. Ionizing radiation insult to the tissue causes DNA damage and free radical formation, which leads to stress-induced programmed cell death-apoptosis. In the long term, this damage leads to bowel obstruction, fistula, perforation, or hemorrhage, and these injuries often require further treatment, in particular, more invasive surgery (22). This study is the first to show that CeO2 nanoparticles confer radioprotection on colon intestinal cells by exerting free radical scavenger properties and SOD mimetic properties.
CeO2 nanoparticles reduce ROS levels and protect normal human colon cells from radiation-induced cell death in vitro
In order to investigate the effects of CeO2 nanoparticles on ROS production, normal human colon cells (CRL 1541) were exposed to increasing concentrations of CeO2 nanoparticles 24 hours prior to a single exposure of 20 Gy radiation. ROS production was measured using the Image-iT LIVETM green ROS detection kit. Results show that when radiation was administered as single therapy, the qualitative production of ROS was significantly increased. However, when CeO2 nanoparticles were administered 24 hours prior to radiation, the presence of CeO2 nanoparticles significantly decreased the ROS production, in a dose-dependent manner (Figure 12A). There was no observable difference in ROS production between the control (non-irradiated cells) and the non-irradiated cells treated in combination with increasing concentrations of CeO2 nanoparticles (Figure 12A) (23).
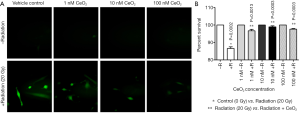
In another set of experiments, normal human colon cells (CRL 1541) were exposed to increasing concentrations of CeO2 nanoparticles added 24 hours prior to a single exposure of 20 Gy. Ninety-six hours later, cell viability was measured. Results show that when radiation was administered as single therapy, the number of viable cells in culture was significantly decreased as compared to control (15%). However, when 1, 10 or 100 nM of CeO2 nanoparticles were administered 24 hours prior to radiation, the CeO2 nanoparticles significantly protected the cells from radiation-induced cell death (3% for 1 nM, 1% for 10 and 100 nM) (Figure 12B) (23).
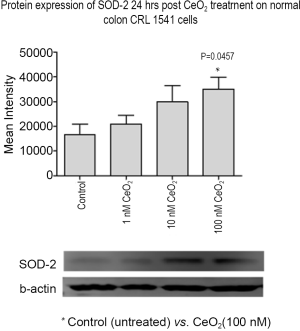
CeO2 nanoparticles induce SOD-2 expression in normal human colon cells in vitro
The effect of CeO2 nanoparticles (added 24 hrs before radiation) on SOD-2 protein expression on CRL 1541 cells growing in normal growth media was measured. Western blot analysis show increased levels of SOD-2 in normal colon cells in the presence of CeO2 nanoparticles and in a dose-dependent fashion, the band intensity of SOD-2 in 100 nM CeO2 nanoparticles treated cells was roughly 2-fold higher than non-treated control cells. The cells exhibited increased SOD-2 expression with the addition of increasing concentrations of CeO2 nanoparticles (Figure 13) suggesting that CeO2 nanoparticles increased normal colon cell SOD-2 expression when added 24 hrs before radiation, conferring cytoprotection from the radiation insult. This phenomenon is corroborated by a corresponding increase in cell survival rates when normal colon cells are treated with increasing doses of CeO2 nanoparticles (23).
CeO2 nanoparticles reduce apoptotic cell death in gastrointestinal mice cells in vivo
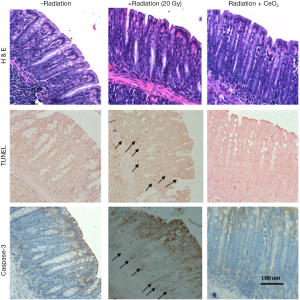
In an attempt to investigate the ability of CeO2 nanoparticles to protect the gastrointestinal epithelium of mice against radiation-induced damage, mice were randomized and colon tissues were harvested and processed four hours post radiation. The colonic crypt cells from mice treated with CeO2 nanoparticles in combination with radiation exhibited a significant decrease in apoptotic colon cryptic cells (as measured by TUNEL) and Caspase-3 expression as compared to the colonic crypt cells from radiated (no CeO2) mice (Figure 14). The number of TUNEL and Caspase-3 positive cells in each colonic crypt decreased by 50% in mice treated with a combination of CeO2 nanoparticles and radiation, as compared to mice treated with radiation alone. It is interesting to note the decrease in Caspase-3 in mice treated with CeO2 nanoparticles as compared to control (normal) mice which could be explained by the fact that CeO2 may reduce the normal intrinsic cell death pathway and/or normal metabolic ROS, as reviewed by Rzigalinksi (7).
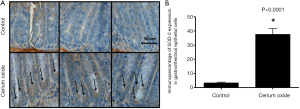
To demonstrate the ability of the CeO2 nanoparticles to induce the overexpression of SOD-2 colons from mice were sectioned 24 hours after a single injection of CeO2 nanoparticles and 10 random crypts per mouse from five different mice per group were stained for SOD-2 expression (Figure 15A). The colonic crypt cells from mice treated with CeO2 nanoparticles exhibited a 40% increase in SOD-2 expression as compared to untreated (normal) mice (Figure 15B). Immunohistochemical analysis of normal colon from mice treated with CeO2 nanoparticles show an increase in SOD-2 expression (23).
Discussion
The field of radiation oncology has worked diligently over the last decade to improve radiation delivery techniques in order to spare sensitive structures from the effects of ionizing radiation. These techniques have resulted in improved functional outcomes compared to prior, more rudimentary, radiation techniques. However, the need to attain adequate tumor coverage and the exquisite radiosensitivity of certain normal structures are intrinsic limitations to the magnitude of function and QOL that can be preserved with these techniques. Hence, even with the implementation of these techniques many patients still experience significant acute and late toxicity after radiation treatment that adversely impacts their QOL.
To further improve radiation-induced toxicities we must continue to develop strategies to protect normal tissues from radiation-induced damage. One such strategy is the development of radiation protectors. Several compounds have been described, but Amifostine remains the only agent currently in clinical use (24). Major limitations to the clinical use of Amifostine are its short half-life, daily dosing requirements, toxicity based on route of administration, and its cost (4,6,15,24). Hence, there remains a substantial clinical need for a radioprotective agent that can be delivered with relative ease, is long lasting, well-tolerated, and can protect a spectrum of sensitive normal tissues that are responsible for a significant reduction in QOL.
The above report lends a great deal of credence to the argument for the use of CeO2 nanoparticles in a therapeutic setting as a free radical scavenger, especially in the context of therapeutic ionizing radiation. As mentioned above, CeO2 nanoparticles, due to their large surface energy derived from a high surface area to volume ratio and unique valence state oscillations, contain many oxygen vacancies which allow them to be much more efficient than endogenous antioxidants, and to be regenerative in their enzymatic activity, which we hypothesize to be due to the valence reversing from +3 to +4 valence states. Additionally, mice administered with CeO2 nanoparticles experience no serious side-effects, demonstrating the low toxicity of CeO2 nanoparticles (15).
Elevated ROS levels have long been implicated in numerous diseases such as kidney fibrosis (25), chronic inflammation and organ dysfunction, especially when induced by ionizing radiation (26). It is now widely accepted that ROS can interfere in intracellular processes which cause the above mentioned injuries. Thus, the therapeutic value of CeO2 nanoparticles may be due to their free radical scavenging properties. Furthermore, CeO2 nanoparticles as scavenging enzymes, are many times more efficient than SOD, which may be due to the large surface area to volume ratio, as well as the ratio of Ce3+/Ce4+ (7). The in vivo experiments also reinforce the conclusion that CeO2 nanoparticles confer significant protection from ionizing radiation as evidenced by TUNEL and Caspase-3 stains, indicators of cell apoptosis (27).
In the end, while CeO2 nanoparticles may affect intracellular oxidative pathways, we show clearly that they are not detrimental; and suspect that the elevated expression of SOD-2 contributes to an increased protection of normal cells against ROS. It is important to note the therapeutic value of free radical scavengers extends beyond protecting against radiation-induced damage to DNA, but also to the reduction in inflammation, fibrosis and organ dysfunction. Thus, we believe that CeO2 nanoparticles are at the forefront of the effort to utilize emerging nanotechnology to improve QOL and healthcare, and that they hold great potential for future clinical trials.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.15). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol 1999;3:77-83. [PubMed]
- Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res 2001;42:21-37. [PubMed]
- Pradhan DS, Nair CKK, Sreenivasan A. Radiation injury repair and sensitization of microorganisms. Proc Indian National Science Academy 1973;39B:516-30.
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev 1998;78:547-81. [PubMed]
- Chen J, Patil S, Seal S, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol 2006;1:142-50. [PubMed]
- Tarnuzzer RW, Colon J, Patil S, et al. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett 2005;5:2573-7. [PubMed]
- Rzigalinski BA, Bailey D, Chow L, et al. Cerium oxide nanoparticles increase the lifespan of cultured brain cells and protect against free radical and mechanical trauma. FASEB J 2003;17:A606.
- Patil S, Sandberg A, Heckert E, et al. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007;28:4600-7. [PubMed]
- Park EJ, Choi J, Park YK, et al. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 2008;245:90-100. [PubMed]
- Gharbi N, Pressac M, Hadchouel M, et al. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 2005;5:2578-85. [PubMed]
- Heckert EG, Karakoti AS, Seal S, et al. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008;29:2705-9. [PubMed]
- Korsvik C, Patil S, Seal S, et al. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb) 2007;1056-8. [PubMed]
- Chen J, Patil S, Seal S, et al. Nanoceria particles prevent ROI-induced blindness. Adv Exp Med Biol 2008;613:53-9. [PubMed]
- Perez JM, Asati A, Nath S, et al. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 2008;4:552-6. [PubMed]
- Colon J, Herrera L, Smith J, et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine 2009;5:225-31. [PubMed]
- Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2000;48:89-94. [PubMed]
- Lee JC, Krochak R, Blouin A, et al. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther 2009;8:47-53. [PubMed]
- Chao KS. Protection of salivary function by intensity-modulated radiation therapy in patients with head and neck cancer. Semin Radiat Oncol 2002;12:20-5. [PubMed]
- Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;50:695-704. [PubMed]
- Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 2000;18:3339-45. [PubMed]
- Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 2009;27:127-45. [PubMed]
- Meissner K. Late radiogenic small bowel damage: guidelines for the general surgeon. Dig Surg 1999;16:169-74. [PubMed]
- Colon J, Hsieh N, Ferguson A, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine 2010;6:698-705. [PubMed]
- Citrin D, Cotrim AP, Hyodo F, et al. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010;15:360-71. [PubMed]
- Kim J, Seok YM, Jung KJ, et al. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol 2009;297:F461-70. [PubMed]
- Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol 2007;80:S23-31. [PubMed]
- Marshman E, Ottewell PD, Potten CS, et al. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J Pathol 2001;195:285-92. [PubMed]


