Improving chemoradiotherapy with nanoparticle therapeutics
Introduction
Nanoparticle (NP) therapeutics are increasingly under investigation and development for cancer treatment (1). NPs possess unique properties, such as preferential accumulation in tumors and low distribution in normal tissue, making them ideally suited for the treatment of tumors. While current preclinical and clinical investigations on NP chemotherapeutics have focused on systemic treatment, a key application of these drugs lies in improving chemoradiotherapy. Chemoradiotherapy has been an important treatment paradigm in oncology. While it has improved survival and disease control, chemoradiotherapy also has significantly higher toxicities when compared to sequential treatment or either treatment alone (2-5). Therefore, there has been strong interest in improving the therapeutic ratio of chemoradiotherapy. One strategy is to improve the delivery of chemotherapy to the tumors while reducing dose to normal tissue. Unfortunately, previous efforts using traditional drug delivery techniques have not been successful (6). However, the recent development of carriers for NP drug delivery offers a unique opportunity. Not only is the biodistribution of NPs well-suited for chemoradiotherapy, their controlled drug release property has the potential to increase the synergy between chemotherapy and radiotherapy, further enhancing therapeutic efficacy. In this review, we will discuss the importance of chemoradiotherapy, why NP therapeutics hold high potential in improving chemoradiotherapy, and preclinical and clinical studies that have evaluated NP therapeutics in chemoradiotherapy.
The chemoradiotherapy paradigm
Over the last three decades, the concurrent administration of chemotherapy and radiotherapy, also called chemoradiotherapy, has emerged as an important treatment paradigm in the curative management of many solid tumors (2). The origin of chemoradiotherapy dates back to 1970s when Nigro and colleagues demonstrated that concurrent radiotherapy and chemotherapy (mitomycin C and 5-fluorouracil) can cure anal cancer without the need for surgery (7). This observation led to the evaluation of chemoradiotherapy for many other cancers. Today, it is part of the standard of care for many difficult to treat cancers, including brain, head and neck, esophageal, gastric, pancreatic, small cell and non-small cell lung, rectal, bladder, anal, vulvar and cervical cancers (2-5,8-14). In chemoradiotherapy, systemically administered chemotherapy not only addresses the potential distant micrometastatic cancers, but also acts synergistically with local radiotherapy to improve the therapeutic efficacy against the primary tumor (2). Because of this synergistic effect, chemoradiotherapy has not only consistently shown improved local tumor control but also improves the rates of cancer cure when compared to either sequential treatment or sole administration of chemotherapy or radiotherapy in large randomized clinical trials (2,3). Furthermore, chemoradiotherapy also allows the sparing of normal organs and omission of surgery in the management of head and neck, anal, bladder and cervical cancers (5,11-14). These organ-sparing approaches have significantly improved the quality of life of cancer patients. Lastly, chemoradiotherapy can also cure patients with esophageal and non-small cell lung cancers who are ineligible for surgical resection due to poor general health (10).
Despite the success of chemoradiotherapy, it is not without limitations. Chemoradiotherapy cannot always eradicate the primary tumor, especially in diseases such as pancreatic cancer. The addition of chemotherapy to radiotherapy has not been able to reduce the dose of radiation needed to achieve high probability of cure. The concurrent use of both chemotherapy and radiotherapy has also significantly increased the toxicity profile of cancer treatment (4,5). Such toxicity has prevented many patients who have poor general health from pursuing chemoradiotherapy. Therefore, there is a need to improve the therapeutic ratio of chemoradiotherapy. This can be accomplished by either increasing the therapeutic efficacy, lowering the toxicity of chemoradiation, or both.
Current approaches to improving the therapeutic index of chemoradiotherapy generally involve the incorporation of molecularly targeted agents. Molecularly targeted agents, such as bevacizumab and cetuximab, have been evaluated in chemoradiotherapy in rectal cancer and head and neck cancer, respectively (15,16). The addition of bevacizumab to chemoradiotherapy for rectal cancer has not shown significant improvement in pathological response or survival. In head and neck cancer, the addition of cetuximab to platinum-based chemoradiotherapy did not improve clinical outcome (17). Thus, there is a strong need for novel approaches and the development of more effective agents that can improve the therapeutic ratio of chemoradiotherapy.
NP properties that are uniquely suited for chemoradiotherapy
NP therapeutic carriers possess several important characteristics that are well-suited for the delivery of agents to improve chemoradiotherapy.
The enhanced permeability and retention (EPR) effect
NPs preferentially accumulate in tumors through the EPR effect, leading to high intratumoral drug concentrations (18,19). Tumor vasculature is generally disorganized, with aberrant branching and leaky walls (20-25). Tumor angiogenesis results in rapid proliferation of endothelial cells and decreased number of pericytes, which leads to porous and leaky blood vessels. These pores can range from 100 nm to several hundred nanometers in diameter, as compared to normal vessel junctions of 5-10 nm (18,21,25). Such large pores leads to higher vascular permeability and hydraulic conductivity in tumors, enabling macromolecules such as NPs to extravasate into tumor interstium (20,21). In normal tissue, macromolecules can be effectively cleared by the lymphatic system. However, tumors have impaired and inefficient lymphatics, which in turn causes the accumulation of NPs. This combination of enhanced of irregular tumor vasculature structure, high vascular density within the tumor, increased tumor vessel permeability, and defective lymphatic drainage, is called the EPR effect (22,24). The effects of high intratumoral drug concentration can be further magnified by radiotherapy, which is spatially targeted to the tumor. Lastly, radiotherapy can also enhance the effects of EPR, leading to more preferential accumulation of NPs at the tumor. Several preclinical studies have shown that NP accumulation is higher in irradiated tumors than non-irradiated tumors (26,27). Thus, NP chemotherapeutics can improve the therapeutic efficacy of chemoradiotherapy, with the potential for higher rates of complete response (CR) and survival. Moreover, a significant increase in therapeutic efficacy can lead to reductions in radiotherapy and chemotherapy doses, which in turn can reduce treatment toxicity.
Unique biodistribution of NP therapeutics
The unique biodistribution of NP therapeutics differs from that of small molecule drugs and is favorable to application in chemoradiation. Due to their macromolecule size, NPs are unable to penetrate normal vasculature and capillaries. Therefore, NP chemotherapeutics generally lead to lower drug dose to normal tissues such as skin, lung, and heart when compared to their small molecule counterparts (22). Given that most of the toxicity from chemoradiotherapy is the result of normal tissue receiving chemotherapy and radiotherapy, the lower drug concentrations in normal tissue provided by NPs can significantly reduce treatment toxicity. Unlike conventional chemotherapy agents that are cleared via multiple routes of excretion from the body, NPs are mainly removed from the circulation via the mononuclear phagocytic system (MPS) and hepatic excretion (28). MPS processing (formerly known as the reticuloendothelial system) may lead to the excretion of NPs in bile or accumulation within the Kupffer cells of the liver and macrophages in the spleen (28,29). The distinctive properties of NP accumulation and clearance enhance the therapeutic ratio by reducing the amount of systemic toxicity experienced compared to small molecule therapeutics (30).
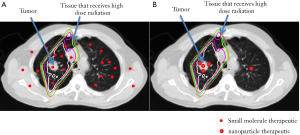
The advantages of NP biodistribution are illustrated in Figure 1. Using chemoradiotherapy for non-small-cell lung cancer (NSCLC) as an example, radiotherapy is aimed at the primary tumor and areas that may harbor macro- and micrometastatic cancers (clinical target volume or CTV). A larger volume of normal tissue is given a significant dose of radiation due to entry and exit of radiation beams and to compensate for motion during treatment of these areas. Since systemically administered small molecule drugs distribute widely in normal organs and tumors, the normal tissue that receives both chemotherapy and radiotherapy can lead to significant toxicities. In contrast, NP therapeutics will mostly concentrate within tumors, leading to improved therapeutic efficacy and lower toxicity.
NP pharmacokinetics and controlled drug release
The pharmacokinetics and drug release properties of NP therapeutics are also favorable for chemoradiotherapy. In general, NPs have longer circulation half-lives and provide higher drug exposure than their small molecule counterparts (31,32). For example, BIND-014, a NP formulation of docetaxel, has an area under the curve (AUC) that is more than 100 times higher than docetaxel at the same dose. In addition, current NP therapeutics under clinical investigation, such as CRLX-101 and BIND-014, release their cargo in a controlled, sustanied release fashion (31,32). Both increased drug exposure and prolonged drug release can increase the synergistic effects between chemotherapy and radiotherapy in tumor cells, improving therapeutic efficacy. Increased drug exposure improving chemoradiotherapy has been demonstrated in a large randomized phase III clinical trial in chemoradiotherapy for rectal cancer (33). Protracted infusional 5-flurouracil (5-FU) improved the therapeutic efficacy and survival when compared to bolus 5-FU. Such data suggest that the use of NP therapeutics over their small molecule counterparts should have similar effects on chemoradiotherapy.
Preclinical and clinical studies evaluating NP therapeutics in chemoradiotherapy
Liposomal therapeutics
Liposomal formulations of doxorubicin were the first NP therapeutics that were developed for the clinical treatment of cancer (34). Several preclinical studies evaluated the use of liposomal doxorubicin in chemoradiotherapy. Harrington et al. were the first to study liposomal doxorubicin and liposomal cisplatin with concurrent radiotherapy in a mouse xenograft model of head and neck cancer (35). The authors demonstrated that liposomal doxorubicin was more effective than doxorubicin in delaying KB tumor growth with both single fraction (4.5 or 9 Gy) and fractionated radiotherapy schedules (9 Gy in 3 fractions). In the study of liposomal doxorubicin in a murine xenograft model of osteosarcoma, Davies et al. also found that liposomal doxorubicin and radiotherapy acted synergistically to enhance the antitumor effect and delay tumor growth (36). Furthermore, the investigators demonstrated that radiotherapy improved the biodistribution of liposomal doxorubicin, with increased tumor uptake of the drug by a factor of two to four after radiotherapy. Such novel findings suggest that the improved distribution is partially responsible for the higher therapeutic efficacy of liposomal doxorubicin.
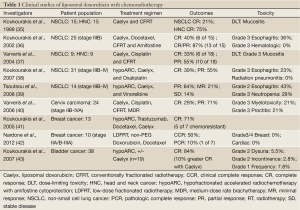
Clinically, liposomal doxorubicin has been evaluated in several early phase clinical trials of chemoradiotherapy. Koukourakis and colleagues conducted most of these studies. His group first reported a Phase I trial on the use of liposomal doxorubicin (Caelyx) with conventionally fractionated radiotherapy for locally advanced NSCLC and head and neck cancer (37). In this small study of 30 patients (15 for each disease), the investigators found 20 mg/m2 of Caelyx was the maximum tolerated dose (MTD) in head and neck cancer chemoradiotherapy and 25 mg/m2 was the MTD for NSCLC chemoradiotherapy treatment. The dose-limiting toxicities were mucositis for head and neck cancer treatment and esophageal toxicity for NSCLC treatment. In a follow-up phase I/II study, patients with inoperable (stage IIIb; T3,4-N2,3-M0) NSCLC were enrolled to receive Taxotere, Caelyx and radiotherapy (38). Patients were also given amifostine to minimize toxicity. Grade 3 and higher esophagitis, which was the dose-limiting toxicity, developed in 9 out of 25 patients (36%). The response rates were 40% CR and 87% partial response (PR), which were encouraging for further evaluation. In addition to above mentioned studies, liposomal formulations of doxorubicin have also been investigated in chemoradiotherapy for head and neck cancer, cervical cancer, recurrent breast cancer and bladder cancer. These clinical studies are outlined in Table 1 (39-45).
Full table
Despite promising results from these small clinical trials, liposomal doxorubicin has not been adopted into chemoradiotherapy treatment. One main reason is that small molecule doxorubicin has not been utilized in chemoradiotherapy for any cancer treatment. Furthermore, while the clinical results from the liposomal doxorubicin are promising, they are not far superior to standard chemoradiotherapy regimens. For example, the response rates of chemoradiotherapy with liposomal doxorubicin are comparable to that of cisplatin with radiotherapy for head and neck cancer.
Liposomal formulations of cisplatin, while not as extensively studied as doxorubicin, have also been investigated in a few preclinical and early-phase clinical trials. Nanoliposome encapsulated cisplatin was found to have a greater radiosensitizer effect in in vitro A549 cells and in vivo Lewis lung carcinoma when compared to cisplatin alone (46). The liposomal formulation of cisplatin, Lipoplatin, was also assessed in combination with radiotherapy for the treatment of F98 glioma orthotopically implanted in Fischer rats. The toxicity of the Lipoplatin was significantly less than cisplatin, though unexpectedly there was lower tumor uptake of the liposomal formulation. This was not the case for Lipoxal, the liposomal formulation of oxaliplatin. Tumor uptake was 2.4-fold more than with the liposome-free oxaliplatin (47). Thus, encapsulating platinum compounds in a liposomal formulation may allow for improved drug delivery to the tumor and reduction of adverse systemic and local effects, thus enhancing the therapeutic ratio and tumor response to treatment with chemoradiotherapy.
Clinical data evaluating liposomal platinum compounds is also emerging. In 20 head and neck cancer patients treated at the University of Pennsylvania, Rosenthal et al. evaluated liposomal cisplatin as a radiosensitizer concurrent with conventionally fractionated radiotherapy. After minimizing transfusion reactions by slowing the infusion rate and increasing the dilution of the formulation, the liposomal cisplatin dose was successfully escalated, with similar treatment-related toxicities to that of concurrent cisplatin. For example, grade 3 skin and mucosal toxicities within the radiation field were minimal, occurring in only 1 and 6 patients, respectively. Furthermore, 11 of the 20 patients (55%) had an initial CR at the primary tumor site after completing treatment (48). In a study of 12 patients with locally advanced gastric cancer who received Lipoplatin, 5-fluorouracil and concurrent radiation therapy, high CR rates and minor toxicities were also observed. CR rates improved from 33% in patients treated with four cycles to 80% in patients treated with five cycles of combined chemoradiotherapy with Lipoplatin, though the total number in each group was small at six and five patients, respectively. Again, the toxicity experienced was comparable with conventional cisplatin, and performance status actually had a net improvement at 2 months from the end of treatment (49). As with liposomal doxorubicin, the early-phase results of Lipoplatin are encouraging and await validation in larger clinical trials.
Polymer drug conjugates
One of the strategies to improve the pharmacokinetics of chemotherapeutics is to conjugate them to hydrophilic polymers to increase their circulation time. These drug-polymer conjugates can be considered NPs as they are nanometers in size. However, drug-polymer conjugates are generally smaller than 10 nm and thus do not possess some of the NP properties, such as controlled release, that are mentioned above. Current drug-polymer conjugate research has centered around the N-(2-hydroxypropyl) methacrylamide (HPMA) polymer and polyethylene glycol (PEG). Several HPMA conjugates, including doxorubicin, camptothecin and palictaxel have been evaluated in early phase clinical trials (50). The results are encouraging. There have been several preclinical reports studying HPMA drug conjugates in chemoradiotherapy. Lammers et al. studied HPMA-doxorubicin and HPMA-gemcitabine conjugates with radiotherapy in ATI rat prostate carcinoma tumor model (26). The investigators found that radiotherapy can increase the tumor accumulation of polymer-drug conjugates. Moreover, the polymer-drug conjugates functioned synergistically with radiotherapy in delaying tumor growth. In a separate report by the same group, the investigators showed that radiotherapy can consistently increase the intratumoral concentration of HPMA copolymers independent of the tumor model (27). Such observations suggested that the improved therapeutic efficacy from nanotherapeutics in chemoradiation can be due to both EPR and radiation induced preferential accumulation. Another polymer-drug conjugate that has been studied clinically is poly(L-glutamic acid)-paclitaxel (PG-TXL). It was evaluated preclinically in chemoradiation by Li et al. in mouse xenograft model of ovarian cancer (51). PG-TXL was found to act synergistically with radiotherapy in controlling tumor growth. This preclinical data lead to two clinical studies evaluating PG-TXL as a radiosensitizer. The first study was phase I trial using paclitaxel poliglumex with concurrent radiotherapy in esophageal and gastric cancer (52). The investigators identified 70 mg/m2/wk as the MTD. In a recently published trial, the investigators studied paclitaxel poliglumex with temozolomide and radiotherapy in high grade glioma (53). Unfortunately, combining temozolomide and paclitaxel poliglumex lead to high rates and prolonged (5 months) hematologic toxicity. Therefore, the proposed treatment regimen is deemed unsafe.
NP albumin-bound paclitaxel
Abraxane, an albumin-bound 130-nm NP containing paclitaxel, is clinically approved for the treatment of metastatic breast cancer (54). This NP therapeutic demonstrated an improved safety profile and increased efficacy, with a 25% increase in overall response to treatment, when compared to paclitaxel alone (55,56). Preclinical data of mice with ovarian or mammary carcinomas treated with Abraxane, radiotherapy, or a combination of both, were designed to assess antitumor efficacy and normal tissue toxicity. The albumin bound paclitaxel improved radiosensitization, lowering the dose to achieve 50% tumor cure from 54.3 to 35.2 Gy. Significantly, there was no increase in normal tissue toxicity to rapidly and slowly proliferating cells. The greatest radioresponsiveness of the tumor to treatment occurred when radiation was given two to three days after Abraxane was administered (57). Currently, a number of phase III chemoradiotherapy clinical trials in lung, esophageal, head and neck, endometrial and cervical cancer are evaluating the concurrent administration of Abraxane with radiotherapy.
Polymeric NP drugs
Current clinical and preclinical research efforts on nanotherapeutics have focused on polymeric NP platforms. Several polymeric NPs have been evaluated clinically with one formulation (Genexol-PM) that’s approved for clinical use (Korea) (58,59). Although none of these polymeric NPs have been studied clinically in chemoradiation, there are several preclinical studies on this approach. Our group has evaluated Genexol-PM, a polymeric micelle formulation of paclitaxel, with external beam radiotherapy in a mouse model of non-small cell lung cancer (60). Genexol-PM is compared to Taxol at equivalent doses of paclitaxel. We found that chemoradiotherapy with Genexol-PM is more effective than that with Taxol in vivo. Moreover, the paclitaxel dose in normal mouse lung 6 hours after Genexol-PM injection is lower than that of Taxol. Lower paclitaxel dose in normal tissue can potentially translate into lower treatment toxicity from chemoradiotherapy. In a similar study, Jung et al. studied polymeric NP formuations of paclitaxel and docetaxel with radiotherapy in mouse models of non-small cell lung cancer. They observed enhanced synergistic effect with reduced survival fraction of A549 cells in vitro and enhanced tumor growth delay in vivo in xenograft mice (61). The author’s group also demonstrated increased radiosensitization with delivery of a molecular targeted docetaxel NP compared to docetaxel alone and NP docetaxel without molecular targeting. Xenograft head and neck cancer mice were used as preclinical model, with folate selected as the targeting ligand because the folate receptor is often overexpressed in head and neck tumors (62). In addition to the finding that NP docetaxel is more effective than docetaxel, we also found that folate-targeted NP docetaxel is more effective as a radiosensitizer than non-targeted NP docetaxel. Such results suggest that molecular targeting, when combined with NPs, can further improve therapeutic ratio of chemoradiotherapy. Currently, there is intense research interest in developing targeted NPs for cancer treatment (63,64). These preclinical studies support the further investigation of Genexol-PM and other polymeric NPs for use in concurrent chemoradiation.
NP delivery of therapeutic radioisotopes
Although nanomedicine research efforts have mainly focused on the delivery of chemotherapeutics, there is growing interest in the delivery of therapeutic radioisotopes using NPs. Recently, several antibody-radioisotope conjugates have been approved for clinical use in cancer (65). Some the studies involving therapeutic radionanoparticles have been theoretical and qualitative in nature. Bouchat et al. showed that radioactive NPs with ~100 Y-90 atoms per NP can substantially increase the biologic effective dose deposited to a solid tumor (66). Hrycushko et al. have modeled liposomes tagged with both beta emitters Re-186 and Re-188 for the post-surgical treatment of breast cancer, demonstrating this to be a viable method of delivering focal radiation (67,68). Recently, Khan et al. demonstrated the antitumor effect of dendrimer NPs carrying therapeutic loads of Au-198. In a melanoma model, tumors of mice injected with the Au-198 NP decreased in size by 45% compared to the untagged NP with no observed toxicity (69). Our own group has described a combined modality NP, ChemoRad NP (70). The NP is composed of a polymeric core with a lipid monolayer shell. It can encapsulate hydrophobic chemotherapies such as docetaxel and chelate therapeutic isotopes such as yttrium. We were able to show that ChemoRad NP containing docetaxel and Y-90 is more effective against ovarian cancer than treatments with small molecule drugs or NPs containing single agent in a ovarian peritoneal metastasis model (71). Another study reported on a liposome particle combining Indium-111 and vinorelbine (72). The agent showed antitumor efficacy in mice with colorectal carcinoma xenografts. A key concern for this strategy is the potential toxicity on the main clearance organ of NPs, the liver. Further preclinical research is needed to validate the safety of delivering therapeutic radioisotopes using NPs.
Summary and future directions
NP therapeutics possess several characteristics that are well suited for application in chemoradiotherapy. Preclinical studies comparing NP-therapeutics to their small molecule counterparts have all shown that NP therapeutics are more effective and potentially less toxic. Thus, there is strong preclinical data to support clinical investigations of nanotherapeutics in chemoradiation treatment. Existing clinical data evaluating NP therapeutics have been conducted with either drugs that are not typically utilized in chemoradiation (doxorubicin) or with NP therapeutics that have not successfully completed clinical development. Current clinical studies involving the next generation of NP-based therapeutics have mainly focused on systemic treatment. Hence, there is a clear need for more clinical trials studying these novel drugs in the chemoradiation context. Such clinical investigations can facilitate the clinical adoption of NP therapeutics as well as improve the current chemoradiation paradigm. There is also a strong need for preclinical research studying NP therapeutics for chemoradiation. Such efforts should focus on identifying the optimal NP properties, such as size, drug release kinetics and pharmacokinetics for applications in chemoradiation. More studies are also needed to identify novel agents, such as wortmannin, that can improve the therapeutic ratio of chemoradiation. Such preclinical data can facilitate the clinical development of NP therapeutics specifically for chemoradiotherapy application.
Acknowledgments
Funding: This work is supported by grants from the University Cancer Research Fund from the University of North Carolina. AZW is also supported by the National Institutes of Health Center for Nanotechnology Excellence Grant U54-CA151652 and National Cancer Institute R01 CA178748.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.04). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang L, Gu FX, Chan JM, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 2008;83:761-9. [PubMed]
- Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm--general principles. Nat Clin Pract Oncol 2007;4:86-100. [PubMed]
- Eifel PJ. Chemoradiotherapy in the treatment of cervical cancer. Semin Radiat Oncol 2006;16:177-85. [PubMed]
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937-44. [PubMed]
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091-8. [PubMed]
- Rasch CR, Hauptmann M, Schornagel J, et al. Intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer 2010;116:2159-65. [PubMed]
- Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17:354-6. [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [PubMed]
- Willett CG, Czito BG. Chemoradiotherapy in gastrointestinal malignancies. Clin Oncol (R Coll Radiol) 2009;21:543-56. [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [PubMed]
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914-21. [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-7; discussion 717-8. [PubMed]
- Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633-40. [PubMed]
- James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 2012;366:1477-88. [PubMed]
- Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 2009;27:3020-6. [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [PubMed]
- Specenier P, Vermorken JB. Cetuximab: its unique place in head and neck cancer treatment. Biologics 2013;7:77-90. [PubMed]
- Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A 1998;95:4607-12. [PubMed]
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010;7:653-64. [PubMed]
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010;7:653-64. [PubMed]
- Noguchi Y, Wu J, Duncan R, et al. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J Cancer Res 1998;89:307-14. [PubMed]
- Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 2000;65:271-84. [PubMed]
- Chauhan VP, Stylianopoulos T, Martin JD, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol 2012;7:383-8. [PubMed]
- Chauhan VP, Stylianopoulos T, Boucher Y, et al. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu Rev Chem Biomol Eng 2011;2:281-98. [PubMed]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393-410. [PubMed]
- Lammers T, Subr V, Peschke P, et al. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br J Cancer 2008;99:900-10. [PubMed]
- Lammers T, Peschke P, Kühnlein R, et al. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery systems. J Control Release 2007;117:333-41. [PubMed]
- Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3:703-17. [PubMed]
- Almeida JP, Chen AL, Foster A, et al. In vivo biodistribution of nanoparticles. Nanomedicine (Lond) 2011;6:815-35. [PubMed]
- O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004;15:440-9. [PubMed]
- Hrkach J, Von Hoff D, Mukkaram Ali M,et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med 2012;4:128ra39.
- Svenson S, Wolfgang M, Hwang J, et al. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J Control Release 2011;153:49-55. [PubMed]
- O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502-7. [PubMed]
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med 2012;63:185-98. [PubMed]
- Harrington KJ, Rowlinson-Busza G, Syrigos KN, et al. Pegylated liposome-encapsulated doxorubicin and cisplatin enhance the effect of radiotherapy in a tumor xenograft model. Clin Cancer Res 2000;6:4939-49. [PubMed]
- Davies Cde L, Lundstrøm LM, Frengen J, et al. Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res 2004;64:547-53. [PubMed]
- Koukourakis MI, Koukouraki S, Giatromanolaki A, et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol 1999;17:3512-21. [PubMed]
- Koukourakis MI, Romanidis K, Froudarakis M, et al. Concurrent administration of Docetaxel and Stealth liposomal doxorubicin with radiotherapy in non-small cell lung cancer: excellent tolerance using subcutaneous amifostine for cytoprotection. Br J Cancer 2002;87:385-92. [PubMed]
- Varveris H, Kachris S, Mazonakis M, et al. Pegulated liposomal doxorubicin and cisplatin given concurrently with conventional radiotherapy: a phase I dose-escalation trial for patients with squamous cell carcinoma of head and neck and lung. Oncol Rep 2004;12:473-81. [PubMed]
- Koukourakis MI, Patlakas G, Froudarakis ME, et al. Hypofractionated accelerated radiochemotherapy with cytoprotection (Chemo-HypoARC) for inoperable non-small cell lung carcinoma. Anticancer Res 2007;27:3625-31. [PubMed]
- Tsoutsou PG, Froudarakis ME, Bouros D, et al. Hypofractionated/accelerated radiotherapy with cytoprotection (HypoARC) combined with vinorelbine and liposomal doxorubicin for locally advanced non-small cell lung cancer (NSCLC). Anticancer Res 2008;28:1349-54. [PubMed]
- Varveris H, Kachris S, Mazonakis M, et al. Phase I/II trial of external irradiation plus medium-dose brachytherapy given concurrently to liposomal doxorubicin and cisplatin for advanced uterine cervix carcinoma. Strahlenther Onkol 2006;182:125-34. [PubMed]
- Koukourakis MI, Manavis J, Simopoulos C, et al. Hypofractionated accelerated radiotherapy with cytoprotection combined with trastuzumab, liposomal doxorubicine, and docetaxel in c-erbB-2-positive breast cancer. Am J Clin Oncol 2005;28:495-500. [PubMed]
- Nardone L, Valentini V, Marino L, et al. A feasibility study of neo-adjuvant low-dose fractionated radiotherapy with two different concurrent anthracycline-docetaxel schedules in stage IIA/B-IIIA breast cancer. Tumori 2012;98:79-85. [PubMed]
- Koukourakis MI, Tsolos C, Touloupidis S. Radical hypofractionated accelerated radiotherapy with cytoprotection for invasive bladder cancer. Urology 2007;69:245-50. [PubMed]
- Charest G, Sanche L, Fortin D, et al. Glioblastoma treatment: bypassing the toxicity of platinum compounds by using liposomal formulation and increasing treatment efficiency with concomitant radiotherapy. Int J Radiat Oncol Biol Phys 2012;84:244-9. [PubMed]
- Zhang X, Yang H, Gu K, et al. In vitro and in vivo study of a nanoliposomal cisplatin as a radiosensitizer. Int J Nanomedicine 2011;6:437-44. [PubMed]
- Rosenthal DI, Yom SS, Liu L, et al. A phase I study of SPI-077 (Stealth liposomal cisplatin) concurrent with radiation therapy for locally advanced head and neck cancer. Invest New Drugs 2002;20:343-9. [PubMed]
- Koukourakis MI, Giatromanolaki A, Pitiakoudis M, et al. Concurrent liposomal cisplatin (Lipoplatin), 5-fluorouracil and radiotherapy for the treatment of locally advanced gastric cancer: a phase I/II study. Int J Radiat Oncol Biol Phys 2010;78:150-5. [PubMed]
- Kopecek J, Kopecková P. HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev 2010;62:122-49. [PubMed]
- Li C, Ke S, Wu QP, et al. Tumor irradiation enhances the tumor-specific distribution of poly(L-glutamic acid)-conjugated paclitaxel and its antitumor efficacy. Clin Cancer Res 2000;6:2829-34. [PubMed]
- Dipetrillo T, Milas L, Evans D, et al. Paclitaxel poliglumex (PPX-Xyotax) and concurrent radiation for esophageal and gastric cancer: a phase I study. Am J Clin Oncol 2006;29:376-9. [PubMed]
- Jeyapalan S, Boxerman J, Donahue J, et al. Paclitaxel poliglumex, temozolomide, and radiation for newly diagnosed high-grade glioma: a Brown University Oncology Group Study. Am J Clin Oncol 2013; [Epub ahead of print]. [PubMed]
- Miele E, Spinelli GP, Miele E, et al. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomedicine 2009;4:99-105. [PubMed]
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794-803. [PubMed]
- Vishnu P, Roy V. Safety and Efficacy of nab-Paclitaxel in the Treatment of Patients with Breast Cancer. Breast Cancer (Auckl) 2011;5:53-65. [PubMed]
- Wiedenmann N, Valdecanas D, Hunter N, et al. 130-nm albumin-bound paclitaxel enhances tumor radiocurability and therapeutic gain. Clin Cancer Res 2007;13:1868-74. [PubMed]
- Lee KS, Chung HC, Im SA, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat 2008;108:241-50. [PubMed]
- Kim DW, Kim SY, Kim HK, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol 2007;18:2009-14. [PubMed]
- Werner ME, Cummings ND, Sethi M, et al. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:463-8. [PubMed]
- Jung J, Park SJ, Chung HK, et al. Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;84:e77-83. [PubMed]
- Werner ME, Copp JA, Karve S, et al. Folate-targeted polymeric nanoparticle formulation of docetaxel is an effective molecularly targeted radiosensitizer with efficacy dependent on the timing of radiotherapy. ACS Nano 2011;5:8990-8. [PubMed]
- Goldberg MS, Hook SS, Wang AZ, et al. Biotargeted nanomedicines for cancer: six tenets before you begin. Nanomedicine (Lond) 2013;8:299-308. [PubMed]
- Kamaly N, Xiao Z, Valencia PM, et al. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev 2012;41:2971-3010. [PubMed]
- Tomblyn MB, Katin MJ, Wallner PE. The new golden era for radioimmunotherapy: not just for lymphomas anymore. Cancer Control 2013;20:60-71. [PubMed]
- Bouchat V, Nuttens VE, Michiels C, et al. Radioimmunotherapy with radioactive nanoparticles: biological doses and treatment efficiency for vascularized tumors with or without a central hypoxic area. Med Phys 2010;37:1826-39. [PubMed]
- Hrycushko BA, Li S, Shi C, et al. Postlumpectomy focal brachytherapy for simultaneous treatment of surgical cavity and draining lymph nodes. Int J Radiat Oncol Biol Phys 2011;79:948-55. [PubMed]
- Hrycushko BA, Gutierrez AN, Goins B, et al. Radiobiological characterization of post-lumpectomy focal brachytherapy with lipid nanoparticle-carried radionuclides. Phys Med Biol 2011;56:703-19. [PubMed]
- Khan MK, Minc LD, Nigavekar SS, et al. Fabrication of {198Au0} radioactive composite nanodevices and their use for nanobrachytherapy. Nanomedicine 2008;4:57-69. [PubMed]
- Wang AZ, Yuet K, Zhang L, et al. ChemoRad nanoparticles: a novel multifunctional nanoparticle platform for targeted delivery of concurrent chemoradiation. Nanomedicine (Lond) 2010;5:361-8. [PubMed]
- Werner ME, Karve S, Sukumar R, et al. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials 2011;32:8548-54. [PubMed]
- Chow TH, Lin YY, Hwang JJ, et al. Diagnostic and therapeutic evaluation of 111In-vinorelbine-liposomes in a human colorectal carcinoma HT-29/luc-bearing animal model. Nucl Med Biol 2008;35:623-34. [PubMed]